Ultrastructural Pathology of Plasma and Endoplasmic Reticulum Membranes of Nerve and Glial Cells: A Review
Abstract
The alteration of nerve cell plasma membranes is reviewed in some
neuropathological conditions. In moderate brain oedema a continuous
plasma membrane is observed but the cytoplasmic membranes, such as
smooth and rough endoplasmic reticulum membranes appear damaged. In
severe oedema, fragmentation of plasma membrane, enlargement and focal
necrosis of rough endoplasmic cisterns and nuclear envelope, detachment
of membrane-bound ribosomes, and reduction of polysome are found.
Shallow and deep invaginations of plasma membrane, and the formation of
endocytic and clathrin-coated vesicles are seen. In astrocyte cells,
areas of focal necrosis and fragmented limiting plasma membrane,
overdistended rough endoplasmic reticulum cisterns with extense
degranulated membrane domains, and vacuoles of smooth endoplasmic
reticulum with necrotic limiting membrane are observed. Oligodendroglia
cells show notably edematous changes featured by lacunar enlargement of
rough endoplasmic reticulum and nuclear envelope, detachment of membrane
bound ribosomes, and discontinuous plasma membrane. Plastic changes and
damage of synaptic membranes are found.
Synaptic vesicle exocytosis and endocytosis at the non-specialized
regions of presynaptic ending limiting membrane are frequently observed
at activated synapses. In severe brain edema, synaptic disassembly
occurs featured by wide separation of pre and postsynaptic membranes,
and loss of peri synaptic astrocytic glial escheatment. Disruption,
fusion and disassembly of interastrocytary gap junctions have also been
observed. The endothelial cell luminal membrane of brain capillaries
undergoes profound activity changes that characterize increased
cerebrovascular permeability, such as increased formation of micro- and
macropinocytotic vesicles, clathrin coated vesicles, and emission of
pseudopods to form endothelial vacuoles. The alterations of nerve cell
plasma membranes and cytomembranes are related with the anoxic-ischemic
conditions of brain parenchyma. The role of free radical and lipid
peroxidation, disturbed energy metabolism, altered metabolic cascades,
glutamate excitotoxicity, haemoglobin toxicity, protein aggregation, and
presence of extracellular oedema fluid are discussed in relation with
the derangement of nerve cells membranes.
Abbreviations: Endoplasmic Reticulum (ER),
Prion Protein (Prp), Parkinson's Disease (PD), Amyotrophic Lateral
Sclerosis (ALS), Unfolded Protein Response (UPR),
Mitochondria-Associated ER Membranes (Mams) Alzheimer's Disease (AD),
Vesicle-Associated Membrane Protein-Associated Protein B (VAPB), Protein
Tyrosine Phosphatase-Interacting Protein 51 (PTPIP51), Nitric Oxide
Synthase (NOS), Mitochondria (M), Neural Cell Adhesion Molecules (NCAM)
Introduction
Over the four past decades substantial progress have been made in
elucidating the mechanisms by which nerve plasma membranes and
cytomembranes are damaged. Nerve cell membrane alterations were earlier
described by Bass [1] in Creutzfeldt- Jacob disease. Significant
enrichment in cholesterol ester was found by [2] In nerve cell membranes
in adrenoleukodystrophy. Disorganization of endoplasmic reticulum
cisterns, formation of lamellar bodies, and hypertrophy of Golgi complex
were described by Malunova and Samoilov [3] in cat cerebral cortex as
early post-anoxic changes. Tissue surrounding hematomas, traumatic
lesions, infective zones, and certain tumours undergo autocatalytic
peroxidation [4], a lipidic disorder which greatly altered membrane
functions. Demyelination-induced plasticity was found by Coria [5] in
the nodal and internodal axolemma in rat lead-induced neuropathy.
Nuclear membrane indentations were reported by Roos [6] in Huntington’s
disease.
Biochemical and morphological changes in nerve cell membranes were
reported by Salvati [7] in experimental allergic encephalomyelitis.
Macromolecular structure of axonal membrane during acute experimental
allergic encephalomyelitis, and myelin deficient rat optic nerve was
described by Black [8] and Waxman [910] found a drop-in cholesterol
content in neuronal plasmalemma after cerebral ischemia. [11]
encountered continuities between the outer nuclear membrane and the
rough endoplasmic reticulum in hyppocampal neurons during
seizure-induced protein synthesis. Membrane damage during situations of
acute or subacute cerebral aggression have been studied using
experimental models [4,1216]. Glial membrane damage of glial axonal
junction after diffusing axonal injury was reported by Maxwell.
Traumatic brain injuries produce damage to nodal axolemma [17] and a
widespread derangement to the neuronal cytoskeleton [18,19] delayed
phospholipids degradation [20], and calpain- mediated spectr in
breakdown leading to ischemic neuronal death. Babu [21] reported several
protein defects in the plasma membrane of neurons and astrocytes in
chronic ethanol treated rats. Dux [22] described disaggregation of
ribosomes as an early sign of histotoxic pathology heralding delayed
neuronal death. Theriault described gap junction remodelling in the rat
spinal cord after acute compression injury. Maxwell [23] found
alterations in the axolemma and myelin sheath in guinea pig optic nerve
after stretch injury. Praproknit [24] described plasma membrane
fragility in dystrophic neuritis of senile plaques in Alzheimer's
disease. Dabrowska-Bouta [25,26] showed alteration of myelin membranes
after chronic lead intoxication in rats.
Torp [27] described association of fibrillar beta amyloid with
neuronal membrane surface in aged dog brains. Similar findings were also
reported by Yamaguchi [28] in hereditary cerebral haemorrhage with
amyloidosis-Dutch type, Alzheimer disease, and non-demented aged
subjects. Haik demonstrated that the putative transmembrane domain of
prion protein induces neurotoxicity and destabilize nerve cell
membranes.
Brain Ischemia and Membrane Damage
Glutamate ecotoxicity-induced damage of plasma membrane occurs in
transient global cerebral ischemia [29] and in traumatic brain injuries
[30] Peroxidative damage to cell membranes occurs following cerebral
ischemia [31-35]. Transient global cerebral ischemia triggers
suppression of protein synthesis, a process controlled by endoplasmic
reticulum function [36-38]. Protein aggregation examined by electron
microscopy and laser-scanning confocal microscopy has been reported
after focal brain ischemia. It has been suggested that nitric oxide may
contribute to ischemia- induced cell injury acting upon endoplasmic
reticulum, calcium homeostasis, protein synthesis, and energy metabolism
[39].
Damaged of nerve cell plasma membranes, cytoskeleton, rough and
smooth endoplasmic reticulum membranes, lysosomal limiting membrane, and
outer and inner mitochondrial membranes have been reported by [41-43]
in moderate and severe edema associated to congenital hydrocephalus,
brain trauma and brain tumors. Recent studies have suggested that
cholesterol, an important component of membranes that controls their
physical properties and functions, plays a critical role in
neurodegenerative diseases. Enrichment of neuronal plasma membrane with
cholesterol protects cortical neurons from apoptosis induced by soluble
oligomers of the Abeta (1-40) peptide. Conversely, cholesterol depletion
renders cells more vulnerable to the cytotoxic effects of the Abeta
soluble oligomers [44]. The binding of Abeta to membrane lipids
facilitates Abeta fibrillation, which in turn disturbs the structure and
function of membranes, such as membrane fluidity or the formation of
ion channels [45].
Recent reports also indicate that dysfunction of endoplasmic
reticulum, which not only mediates proteins processing, but also
regulates intracellular calcium homeostasis and cell death signal
activation, occurs at an early stage after ischemia, and might be the
initial step of apoptotic cascades in neurons [46]. Singleton and
Povlishock [47] reported plasma membrane disruption in diffuse brain
injury. Luo and Shi [48] have found that acrolein, a by-product of
oxidative stress and lipid peroxidation, inflicts severe axolemmal
disruption. The membrane damage is likely mediated by reactive oxygen
species and lipid peroxidation, which are elevated after acrolein
exposure. Shi has also reported axolemmal disruption in guinea pig
spinal cord following compression. Kurnellas [49] have described plasma
membrane calcium ATPase deficiency in multiple sclerosis and spinal cord
injury, as a potential mechanism of neurodegeneration. Farkas [50]
demonstrated mechanoporation or disruption of neuronal plasma membrane
induced by diffuse traumatic brain injury.
Thompson [51] demonstrated opening of neuronal gap junction
hemichannels following ischemia after stroke. Yi have found an increase
in complexing I and complexing II, considered respectively markers of
inhibitory and excitatory synapses, after traumatic brain injury. Nitric
oxide and its toxic metabolite, peroxynitrite, can inhibit components
of the mitochondrial respiratory chain leading to cellular energy
deficiency and, eventually, to cell death. Kynurenine metabolic pathway,
its alterations and their potential association with cellular energy
impair certain neurodegenerative diseases. During energy production,
most of the O2 consumed by the mitochondria is reduced fully to water,
but 1-2% of the O2 is reduced incompletely to give the superoxide anion
O2- If the function of one or more respiratory chain complexes is
impaired for any reason, the enhanced production of free radicals
further worsens the mitochondrial function by causing oxidative damage
to macromolecules, and by opening the mitochondrial permeability
transition pores thereby inducing apoptosis [52]. Prion protein (PrP),
normally a cell surface protein, has been detected in the cytosol of a
subset of neurons. The appearance of PrP in the cytosol could result
from either retro-translocation of misfolded PrP from the endoplasmic
reticulum (ER) or impaired import of PrP into the ER [53].
Endoplasmic Reticulum Stress and Mitochondria Interplay and the Pathogenesis of Neurodegeneration
Endoplasmic reticulum and mitochondria are in a close communication,
establishing a dynamic ER-Ca2+-mitochondria interconnection that can
play a prominent role in the neuronal cell death induction under
stressful circumstances of Parkinson's disease pathology (PD). Also,
endoplasmic reticulum (ER) stress in conjunction with abnormal protein
degradation can contribute to the PD pathophysiology, [54]. Endoplasmic
reticulum stress, initiated by the accumulation of unfolded or misfolded
proteins, activates the unfolded protein response, which adapts cells
to the stress. If this adaptive response is insufficient, the unfolded
protein response activates an apoptotic program to eliminate the
affected cells- Endoplasmic reticulum stress in myelinating cells is
important in the pathogenesis of various disorders of myelin, including
Charcot-Marie-Tooth disease, Pelizaeus-Merzbacher disease and Vanishing
White Matter Disease, as well as in the most common myelin disorder,
multiple sclerosis [55].
Amyotrophic lateral sclerosis (ALS) is a devastating
neurodegenerative disease characterized by the misfolding and
aggregation of distinct proteins in affected tissues, however, the
pathogenic cause of disease remains unknown. Recent evidence indicates
that endoplasmic reticulum (ER) stress plays a central role in ALS
pathogenesis. ER stress activates the unfolded protein response (UPR), a
homeostatic response to misfolded proteins. The UPR is initially
protective by up-regulation of specific ER stress- regulated genes and
inhibition of general protein translation. However, long-term ER stress
leads to cell death via apoptotic signaling, thus providing a link to
neurodegeneration [56]. a-synuclein pathology and its effects on diverse
protein partners and specific cellular pathways in the membrane and/or
cytosolic districts, such as endoplasmic reticulum/Golgi, axonal and
synaptic compartments of dopaminergic neurons, may cause the onset of
neuronal cell dysfunction and degeneration which are among the key
pathological features of the Parkinson's disease (PD) brain. Besides,
a-synuclein aggregation may induce dysfunction and degeneration of
synapses via these multiple mechanisms [57].
According to Hedskog [58], it is well-established that sub
compartments of endoplasmic reticulum (ER) are in physical contact with
the mitochondria. These lipid raft-like regions of ER are referred to as
mitochondria-associated ER membranes (MAMs), and they play an important
role in, for example, lipid synthesis, calcium homeostasis, and
apoptotic signaling. Perturbation of MAM function has previously been
suggested in Alzheimer's disease (AD). Our data suggest an important
role of ER-mitochondria contacts and cross-talk in AD pathology.
α-Synuclein is located in mitochondrial-associated endoplasmic
reticulum membranes. PD-related mutated a-synuclein results in its
reduced association with mitochondria-associated membranes, coincident
with a lower degree of apposition of endoplasmic reticulum with
mitochondria and an increase in mitochondrial fragmentation. Aging is
associated to cognitive decline and susceptibility to neuron death, two
processes related recently to subcellular Ca2+ homeostasis. Memory
storage relies on mushroom spines stability that depends on
store-operated Ca2+ entry (SOCE). In addition, Ca2+ transfer from
endoplasmic reticulum (ER) to mitochondria sustains energy production
but mitochondrial Ca2+ overload promotes apoptosis. We have addressed
whether SOCE and ER-mitochondria Ca2+ transfer is influenced by culture
time in long-term cultures of rat hippocampal neurons, a model of
neuronal aging [59].
However, many neuronal functions damaged in Parkinson's disease are
regulated by signaling between the endoplasmic reticulum (ER) and
mitochondria. This signaling involves close physical associations
between the two organelles that are mediated by binding of the integral
ER protein vesicle-associated membrane protein-associated protein B
(VAPB) to the outer mitochondrial membrane protein, protein tyrosine
phosphatase-interacting protein 51 (PTPIP51). VAPB and PTPIP51 thus act
as a scaffold to tether the two organelles. a-synuclein induced
loosening of ER-mitochondria contacts is accompanied by disruption to
Ca2+ exchange between the two organelles and mitochondrial ATP
production. Such disruptions are likely to be particularly damaging to
neurons that are heavily dependent on correct Ca2+ signaling and ATP
[60].
The Role of Calcium Overload
Potentially toxic cytoplasmic calcium concentrations can also occur
due to release from internal stores, either through physical damage to
mitochondria and the endoplasmic reticulum, or a malfunction of
receptors and channels present in their membranes. Such increases of
cytoplasmic calcium concentrations can trigger a range of downstream
neurotoxic cascades, including the uncoupling mitochondrial electron
transfer from ATP synthesis, and the activation and overstimulation of
enzymes such as calpains and other proteases, protein kinases, nitric
oxide synthase (NOS), calcineurin and endonucleases [61].
Autophagy and Apoptosis
Autophagy and apoptosis are basic physiologic processes contributing
to the maintenance of cellular homeostasis. Autophagy encompasses
pathways that target long-lived cytosolic proteins and damaged
organelles. It involves a sequential set of events including double
membrane formation, elongation, vesicle maturation and finally delivery
of the targeted materials to the lysosome. Deregulation of autophagy
plays a pivotal role in the etiology and/ or progress of many
neurodegenerative disorders [62]. Neurons employ specialized mechanisms
to modulate local gene expression in dendrites, via the dynamic
regulation of micro RNA biogenesis factors at intracellular membranes of
the endoplasmic reticulum, which in turn is crucial for neuronal
dendrite complexity and therefore neuronal circuit formation and
function.
In the present review we describe the ultrastructural alteration of
nerve cell and glial plasma membrane, cytomembranes, synaptic membranes,
and interastrocytary gap junctions in cortical biopsies of patients
with vascular anomalies, congenital hydrocephalus, complicated brain
traumatic injuries. The brain parenchyma of these patients exhibits
moderate and severe oedema and sustained anoxic-ischemic conditions
[63-66].
Alterations of Plasma Membrane and Cytomembranes in Vascular Anomaly and Moderate Oedema
In vascular anomaly, the perifocal cerebral cortex exhibits a
microstructure like that observed in normal animal brain cortex. The
plasma membrane of non-pyramidal neurons shows in moderate oedema atly
normal and continuous structure. The plasma membrane form endocytic and
clathrin coated vesicles internalizing toward the cytoplasm. The
limiting plasma membrane of rough endoplasmic reticulum cisterns exhibit
fragmented areas and detachment of membrane associated ribosomes. The
nuclear envelope appears irregularly dilated (Figures 1 & 2).
Disaggregation of ribosomes was earlier demonstrated by Dux in primary
cortical and hippocampal neuronal cultures after brief histotoxic
hypoxia.
Figure 1: Anomaly of anterior cerebral artery. Right
pa-rietal cortex. Non-pyramidal nerve cell showing an irreg-ularly
dilated nuclear envelope (short arrows), enlarged endoplasmic reticulum
cisterns (ER), detachment of mem-brane associated ribosomes (long
arrows), and a contin-uous limiting plasma membrane (arrowheads). Note
the prominent nucleus (N), the nucleolus (NL), the swollen mitochondria
(M), and the non-dilated extracellular space in the neighboring neuropil
that features moderate brain edema.
Figure 2: Anomaly of anterior cerebral artery. Right pari-etal
cortex. Non-pyramidal neuron (NP) showing shallow invaginations of
plasma membrane (long arrow) and for-mation of clathrin coated vesicle
(arrowhead). Endocytic vesicles are also observed in sublemmal
localization short arrows). Note the disrupted and degranulated rough
en-doplasmic reticulum cisterns (ER).
Membrane Abnormalities in Congenital Hydrocephalus
In relationship with congenital hydrocephalus in neonate patients,
the presence of interstitial oedema fluid in the enlarged extracellular
space of an immature neuropil induces fragmentation of plasma membrane,
lacunar enlargement of rough endoplasmic reticulum, detachment of
membrane associated ribosomes, irregularly dilated nuclear envelope, and
nuclear pore disassembly [67] (Figure 3). Congenital hydrocephalus.
Right frontal cortex. Non-pyramidal neuron exhibiting fragmented plasma
membrane (long arrow), distended endoplasmic reticulum cisterns (ER),
detachment of membrane associated ribosomes (short arrows), and
disassembly of some nuclear pores (arrowheads). The mitochondria (M)
appear swollen. Note the enlarged extracellular space in the
neighbouring neuropil (asterisks). X 60.000.
Figure 3: Congenital hydrocephalus. Right frontal cortex.
Non-pyramidal neuron exhibiting fragmented plasma membrane (long arrow),
distended endoplasmic reticu-lum cisterns (ER), detachment of membrane
associated ri-bosomes (short arrows), and disassembly of some nuclear
pores (arrowheads). The mitochondria (M) appear swol-len. Note the
enlarged extracellular space in the neigh-bouring neuropile (asterisks).
X 60.000.
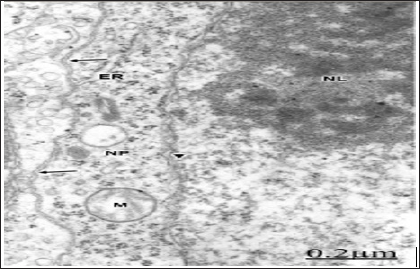
The plasma membrane shows deep invaginations and formation of
numerous endocytic vesicles directed toward the endoplasmic reticulum
and the Golgi apparatus [68]. The lysosomes show fragmented limiting
membrane (Figure 4). In congenital hydrocephalus in neonate patients we
are dealing with immature plasma membranes, characterized by changes in
the integral membrane proteins, cholesterol domains, and in certain
carbohydrates residues and anionic sites [69]. These different molecular
compositions of immature plasma membranes explain its high sensitivity
to injury factors. In hypertensive congenital hydrocephalus, the
pressure exerted by the non-circulating cerebrospinal fluid induces
plasma membrane fragmentation, and enlargement of intracellular nerve
cell compartments. In addition, the presence of oedematous mitochondria
evinces a disturbed energy metabolism.
Endocytosis is a fundamental mechanism by which neurons control
intercellular signal, nutrient uptake, and synaptic transmission [70].
Clathrin-coated vesicles of different sizes have been isolated from rat
brain, and related with the content of Na+, K (+)- ATPase, as a
mechanism of Cl- uptake [71], as pathways for endocytosis of neural cell
adhesion molecules (NCAM) [72], and for receptor-mediated vesicular
transport [73], such as internalization of metabotropic glutamate
receptors [74].
Plasma Membrane Damage in Human Severe Traumatic Brain Oedema
In patients with brain trauma and moderate oedema, some non-pyramidal
neurons show a well preserved and continuous plasma membrane, swollen
mitochondria [75,76] (Figure 5). In severe oedema of traumatic brain
injuries complicated with subdural hematoma, the non-pyramidal neurons,
astrocytes and oligodendrocytes show plasma membrane fragmentation,
swollen mitochondria, enlargement of rough and smooth endoplasmic
reticulum cisterns, and irregular dilation of nuclear envelope [77]. The
rough endoplasmic reticulum displays extensive areas with detachment of
membrane-bound associated ribosomes, and a marked reduction in the
number of polysomes [78] (Figure 6).
Figure 5: Brain trauma. Left frontal hematoma. Non-py-ramidal
neuron in an area of moderate perifocal oede-ma bearing a continuous
plasma membrane (arrows), a non-dilated endoplasmic reticulum cistern
(ER) and nucle-ar envelope (arrowhead), and swollen mitochondria (M).
Note the well-preserved nucleolar substructures (NL).X 30.000.
The plasma membrane shows deep invaginations and formation of
numerous endocytic vesicles directed toward the endoplasmic reticulum
and the Golgi apparatus [68]. The lysosomes show fragmented limiting
membrane (Figure 4). In congenital hydrocephalus in neonate patients we
are dealing with immature plasma membranes, characterized by changes in
the integral membrane proteins, cholesterol domains, and in certain
carbohydrates residues and anionic sites [69]. These different molecular
compositions of immature plasma membranes explain its high sensitivity
to injury factors. In hypertensive congenital hydrocephalus, the
pressure exerted by the non-circulating cerebrospinal fluid induces
plasma membrane fragmentation, and enlargement of intracellular nerve
cell compartments. In addition, the presence of oedematous mitochondria
evinces a disturbed energy metabolism.
Endocytosis is a fundamental mechanism by which neurons control
intercellular signal, nutrient uptake, and synaptic transmission [70].
Clathrin-coated vesicles of different sizes have been isolated from rat
brain, and related with the content of Na+, K (+)- ATPase, as a
mechanism of Cl- uptake [71], as pathways for endocytosis of neural cell
adhesion molecules (NCAM) [72], and for receptor-mediated vesicular
transport [73], such as internalization of metabotropic glutamate
receptors [74].
Plasma Membrane Damage in Human Severe Traumatic Brain Oedema
In patients with brain trauma and moderate oedema, some non-pyramidal
neurons show a well preserved and continuous plasma membrane, swollen
mitochondria [75,76] (Figure 5). In severe oedema of traumatic brain
injuries complicated with subdural hematoma, the non-pyramidal neurons,
astrocytes and oligodendrocytes show plasma membrane fragmentation,
swollen mitochondria, enlargement of rough and smooth endoplasmic
reticulum cisterns, and irregular dilation of nuclear envelope [77]. The
rough endoplasmic reticulum displays extensive areas with detachment of
membrane-bound associated ribosomes, and a marked reduction in the
number of polysomes [78] (Figure 6).
Figure 5: Brain trauma. Left frontal hematoma. Non-py-ramidal
neuron in an area of moderate perifocal oede-ma bearing a continuous
plasma membrane (arrows), a non-dilated endoplasmic reticulum cistern
(ER) and nucle-ar envelope (arrowhead), and swollen mitochondria (M).
Note the well-preserved nucleolar substructures (NL).X 30.000.

Concluding Remarks
In moderate brain oedema a continuous plasma membrane is observed in
some neurons, but the cytoplasmic membranes, such as smooth and rough
endoplasmic reticulum membranes appear damaged. In severe oedema,
fragmentation of plasma membrane, enlargement and focal necrosis of
rough endoplasmic cisterns and nuclear envelope, detachment of
membrane-bound ribosomes, and reduction of polysome are found [118].
Shallow and deep invaginations of plasma membrane, and the formation of
endocytic and clathrin-coated vesicles are seen. In astrocyte cells,
areas of focal necrosis and fragmented limiting plasma membrane,
overdistended rough endoplasmic reticulum cisterns with extense
degranulated membrane domains, and vacuoles of smooth endoplasmic
reticulum with necrotic limiting membrane are observed. Oligodendroglia
cells show also notably edematous changes featured by lacunar
enlargement of rough endoplasmic reticulum and nuclear envelope,
detachment of membrane bound ribosomes, and discontinuous plasma
membrane [119-121]. Plastic changes and damage of synaptic membranes are
found. Synaptic vesicle exocytosis at the synaptic active zone, and
endocytosis at the non-specialized regions of presynaptic ending
limiting membrane are frequently observed at activated synapses.
In severe brain edema, synaptic disassembly occurs featured by wide
separation of pre- and postsynaptic membranes and loss of peri synaptic
astrocytic glial escheatment. Disruption, fusion and disassembly of
interastrocytary gap junctions have also been observed. The endothelial
cell luminal membrane of brain capillaries undergoes profound activity
changes that characterize increased cerebrovascular permeability, such
as increased formation of micro- and macropinocytotic vesicles, clathrin
coated vesicles, and emission of pseudopods to form endothelial
vacuoles, The alterations of nerve cell plasma membranes and
cytomembranes are related with the anoxic-ischemic conditions of brain
parenchyma [121-124]. The role of free radical and lipid peroxidation,
disturbed energy metabolism, altered metabolic cascades, glutamate
excitotoxicity, haemoglobin toxicity, protein aggregation, and presence
of extracellular oedema fluid are discussed in relation with the
derangement of nerve cells membranes.
Is Laser the Best Energy Source to Perform Thermal Ablation?- https://biomedres01.blogspot.com/p/blog-page_4.html
More BJSTR Articles : https://biomedres01.blogspot.com





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.