Natural H-Clinoptylolite in Synthesis & in Waste Water Treatment From of Acetoaminophen
Introduction
Here is presented the results about the acylation of p-aminophenol adsorbed from an aqueous solution on H-clinoptilolite with acetic anhydride. It is known that the acylation of p-aminphenol is carried out with acetic anhydride or acetic acid chloride in acidic media [2,3]. It is assumed that a convenient reagent medium for this process is the use of heterogeneous acid catalytic systems based on aluminosilicates [2]. Nowadays natural zeolites are focused to applications in the sector of wastewater decontamination. There are many reasons for zeolites using in mentioned fields: good selectivity for many toxic cations and harmful compounds [4,5], as adsorbents for organic compounds [5]. Zeolites occur in nature in specific kinds of rocks. Zeolite rich rocks are widespread in Northern part of Armenia, occurring in much extended geological formations. The zeolite types are exclusively clinopilolite - in Idjevan / Northern-East of Armenia.
Discussion
Acylation proceeds by the mechanism of nucleophilic addition, in which the nitrogen atom of p-aminophenol as a nucleophile, attacking the carbonyl group of acetic anhydrides. An intermediate product is formed, which undergoes further elimination of the acetate anion. It is known that phenol is adsorbed better than aniline on the surface of H-zeolites. This probably also takes place during the deposition of aminophenol on the surface of the zeolite, thereby facilitating the reaction of chemioselective N-acylation of the free nitrogen atom in the molecule when reacting with acetic anhydride. A comparative analysis of the yield and reaction time during the synthesis of acetaminophen by the traditional method and by the method using H-zeolite as a heterogeneous catalyst was carried out. In the first case, the yield of paracetamol per hour is 86% [1], and when using the proposed method, 75% per hour. However, this method assumes that the reaction is carried out in the absence of a solvent, as well as the ease of isolating the reaction product. An optimal conditions have been established in order to isolate the product by means of a crystallization for improving the yield. The process of recrystallization of the product from an ethanol solution was also carried out to obtain a pure, white color of the synthesized acetaminophen. A study of the solvent system for thin-layer chromatography (TLC) of acetaminophen was also carried out. The most successful was the system of solvents - ethyl acetate: hexane - 1:1.
Kinetics Study for Acetaminophene Sorption on Zeiolites
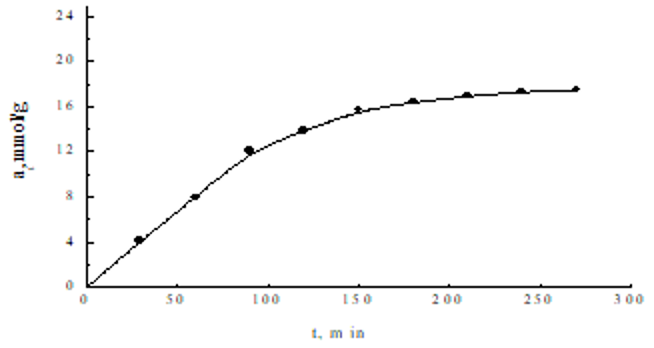
In Figure 1 the adsorption of acetaminophene on zeolite depending of contact time is presented. It was obtained that the uptake of increases with the lapse of time. Absorption of acetaminophen is rapid in the first 100 minutes, after which the rate slows to equilibrium. The test results can be used to study the speed limit step.
Experimental Part
Synthesis of acetaminophen was carried out on H-clinoptilolite. The progress of the reaction was monitored by thin layer chromatography (TLC), which was carried out on Silufol UV-254, and the development of trace substances was carried out in a desiccator saturated with iodine vapor. Gas-liquid chromatography was carried out on a GLC instrument, Cristall-2000 M, by using polar liquid phases DB-5 in the laboratory. Infrared (IR) spectra were recorded on a Specord IR 75 spectrophotometer. NMR spectra were recorded on a Varian USA Mercury Plus 〖NMR〗^1H spectrophotometer (300 MHz).
Preparation of Zeolites: Samples of natural zeolites - clinoptilolite and mordenite, previously crushed to sizes <10 mm, were subjected to grinding in a laboratory ball mill, then the crushed zeolites were subjected to sieve classification. Samples of natural zeolites purified from impurities were dried to constant weight at 105°C, a fraction of 0.25–0.5 mm was isolated by scattering, from which moisture was additionally removed at 350°C. Before weighing, the samples were kept in a desiccator over calcium chloride. Clinoptilolite was transferred to the H-form by treatment with 10% hydrochloric acid, at a ratio of T:L = 1:10 and room temperature (25°C), for 3 hours, then the zeolite was washed from acid on the filter until a negative reaction to the Cl- ion and dried.
Synthesis of Paracetamol by Acetylation of p-aminophenol on H-clinoptilolite: 11 g (0.1 mol) of p-aminophenol and 13 g (0.127 mol) of acetic anhydride pre-adsorbed on 25 g of H-clinoptilolite were stirred at room temperature in the absence of a solvent. The course of the reaction was monitored using a gas-liquid chromatograph ( GLCh - Crystal 2000M ). Heating in a boiling water bath lasted 1 hour. The mixture was extracted with CH2Cl2 (2×120 ml) and the organic layers were separated, washed with saturated NaHCO3 (2×150 ml) and water (100 ml) and dried over anhydrous MgSO4. Crystals began to form after filtering the solution. After separation of the crystals, the reaction mixture was filtered for separation the crystals. The filtrate was washed with cold water (+8, + 10˚C) and dried on filter paper. Then the filtrate was placed in a dryer /at a temperature of 100°C/. The resulting powder had a light pink color, so it was recrystallized in vacuo. Yield 11.34 g (75%) acetaminophen m.p. 167-170°C.
The identity of the product was confirmed on a gas-liquid chromatograph with a flame ionization detector under the following conditions: column, capillary column DB-5 (polydimethylsiloxane) for GC - size 0.30 m x 25 mm, Detector - FID; The mobile phase is nitrogen; column temperature 100°C; detector temperature 240°С; evaporator temperature 260°C. Retention time of paracetamol 2.40 min. The obtained crystals were dissolved in 70% ethanol (30 ml) for recrystallization. The solution was then heated to 60°C., then left to cool before bringing the temperature in the flask to room temperature, and again immersed in ice water for recrystallization. The crystallized solution was filtered and dried. The second time the crystals turned white. After recrystallization, 10.95 g (0.072 mol) of acetaminophen was isolated.
The identification of the obtained compound of the substance was carried out by joint thin-layer chromatography with the initial aminophenol. It was found:
1. Eluent ethyl acetate: hexane 1:1-Rf for acetaminophen is 0.62, Rf - 0.74 for p-aminaphenol,
2. Eluent chloroform: acetone 5:1-R for acetaminophen is 0.32, Rf is 0.50 for p-aminaphenol,
3. Eluent cyclohexane: acetone 1:1 - Rf for acetaminophen is 0.60, Rf is 0.82 for p-aminaphenol.
Identification of acetaminophen (paracetamol) was also carried out by a known method by GLC using polar liquid phases DB-5. IR spectrum, ν, cm-1: s 1660 - 1560 (aromatic ring, C=O, N-H), br 3325 - 3150 (N-H).
1H NMR spectrum, δ, ppm: 1.9 s (3H CH3), 6.70 (2H, Harom, J 8.2 Hz), 7.65 d (2H, arom, J 8.2 Hz), 9.15 s (1H OH), 9.70 br.s (1H NH).
Natural zeolites - clinoptilolite were dehydrated at 400oC in vacuum (0.1 mm) for 3-4 hours. H- clinoptilolite obtained by treatment natural clinoptilolite in HCL [5]. The removal of acetaminophen is carried out as follows. The researches were spent in static conditions on a laboratory rocking chair. H-Clinoptilolite brought in quantity of 1, 0 ± 0.01 g in water solutions, volume 100 ± 0.1 ml containing acetaminophen in quantity from the maximum solubility. Further a mix placed on a rocking chair and subjected to hashing during 6h., at temperature of 20oC, and then test defended within 24 h.
Conclusion
1. The possibility of synthesizing acetaminophen (paracetamol) from p-aminophenol adsorbed on H-clinoptilolite with acetic anhydride in the absence of a solvent has been established.
2. A convenient TLC solvent system was found to establish the identity of acetaminophen.
3. H-clinoptilolite show adsorption activity when paracetamol is removed from an aqueous solution.
For more Articles on: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.