Blighia unijugata (Sapindaceae), a species widespread in tropical and equatorial regions of Africa has been widely used for its multiple therapeutic properties [1]. In Côte d’Ivoire, Nigeria and Congo, this plant is abundantly consumed as vegetables and also used in the treatment of fever, nausea and vomiting, leprosy, eye pain, cough, migraines, rheumatism, kidney pain and joint stiffness, dizziness and especially high blood pressure [2,3]. However, despite the increased use of this medicinal plant, data-t-on its efficacy and safety are not yet available, thus exposing populations to all kinds of dangers. However, scientific studies and the rational evaluation of plants commonly used in traditional medicine could guarantee their best use, reduce the risk of accidents and allow the establishment of specific treatments for its poisonings [4]. Blighia unijugata has been the subject of several scientific publications due to its multiple uses in traditional medicine [5,6]. However, to our knowledge, very few scientific studies have been undertaken to establish the risks of toxicity of this plant on biochemical blood parameters. Previous work in our laboratory has shown that the butanolic fraction of Blighia unijugata (BFBu) has a significant hypotensive effect in rabbit of the species Oryctolagus cuniculus (Leporidae) compared to the other four fractions from the total ethanolic extract of this plant [7]. This study was therefore carried out to evaluate the effects of this active fraction on serum biochemical parameters of male Wistar rats.
Materials
Plant Material: The plant material consisted of powdered leaves of Blighia unijugata. The fresh leaves were collected in Abidjan (Cocody) in June 2009. This species was identified by us using available herbaria and a book written by [8]. The confirmation was made by the National Floristic Center (NFC) of Félix Houphouët-Boigny University (Abidjan, Ivory Coast) where a herbarium specimen has been kept under voucher no165.
Animals: The experiments were carried out on 24 albino rats (Ratus norvegicus), healthy male, of Wistar strain, aged three months. Their average body weight was 148.00±7.07 g. These animals came from the animal facility of the Institut Pasteur de Côte d’Ivoire located in Adiopodoumé (Abidjan) and were reproduced in the animal facility of the Laboratory of the Ecology Research Center of Nangui Abrogoua University (Côte d ‘Ivoire) at an ambient temperature of 25±3 °C. The photoperiod was 12 hours of light and 12 hours of darkness. Water and pellet food (Ivograin®) were fed ad libitum to the rats before the experiment began. These animals were treated according to good laboratory practices [9].
Technical Material : The extraction equipment for the total ethanolic extract and fractions of Blighia unijugata consisted of an electric grinder (Culatti, France), an electronic scale (Mettler Toledo, PB 153-L, Switzerland), a magnetic stirrer (Stuart SB 162, UK), Buchner funnel, cotton wool and Wattman no1 filter paper, Erlenmeyer flask, 500 mL conical flask, rotary evaporator (Büchi R110, Germany) and an oven (Retsch, Germany). The subacute toxicity study was performed using a gastric tube suitable for gavage of rats, pastor pipettes, and dry tubes. The analysis of the biochemical parameters was carried out using an automatic device (KENZA MAX Biochemis Try, France).
Solvents : The total ethanolic extract, hexanic, chloroform, ethyl acetate, butanolic and aqueous fractions of Blighia unijugata were obtained by extractions with different solvents (distilled water, 96% ethanol, hexane, chloroform, ethyl acetate and n-butanol).
Methods
Preparation of the Total Ethanolic Extract of Blighia Unijugata : The total ethanolic extract of Blighia unijugata leaves was prepared according to the method of some authors [10]. The fresh leaves were thoroughly washed with tap water and then dried at room temperature. They were sprayed using an electric grinder (Culatti, France). Cold maceration was carried out with 100 g of powder in 2 liters of 96% ethanol, for 48 h, with magnetic stirring. The resulting solution was first filtered through Buchner and then through Wattman No1 filter paper. This operation was repeated twice on the same powder residue. The filtrates obtained were added and concentrated under reduced pressure at 45 °C using a rotary evaporator (Büchi R110, Germany). The concentrated product, obtained after evaporation, was collected in a container and dried in an oven (Retsch, Germany) at 45 °C for 48 h according to the method described by [11]. The 13.3 g paste obtained corresponds to the total ethanolic extract (TEE) of Blighia unijugata. It was stored at -5 °C in a hermetically sealed jar.
Preparation of Fractions of the Total Ethanolic Extract of Blighia Unijugata : Different fractions of the total ethanolic extract (TEE) were obtained by successive liquid-liquid extractions, with four solvents of increasing polarities (hexan, chloroform, éthyl acétate and n-butanol) according to the methods of [12,13]. Ten grams of the TEE was dissolved in 200 mL of hot distilled water (45 °C). The whole was homogenized by magnetic stirring for 15 min and then it was filtered. Aqueous filtrate obtained was exhausted for 10 min at 27±2 °C with 200 mL of hexane, thus giving two phases after decantation (hexan phase and residual aqueous phase). Residual aqueous phase was again treated for 10 min at 27±2 °C with 200 mL of chloroform to in turn give two phases (chloroform phase and residual aqueous phase). Same operation was successively treating the residual aqueous phase with ethyl acetate to also give an ethyl acetate phase and residual aqueous phase, then with n-butanol to finally obtain butanol phase and residual aqueous phase after settling. Each organic phases and the residual aqueous phase obtained was recovered and concentrated under reduced pressure throught a rotary evaporator (Büchi R110, Germany). Various concentrated products obtained were collected in containers and dried in an oven (Retsch, Germany) at 45°C for 24 h according to the method of some authors [14]. Thus, hexan (0.6 g), chloroform (1.1 g), ethyl acetate (1.6 g), butanol (3.18 g) and aqueous (3.12 g) fractions were obtained. These end products were tested on rabbit blood pressure in the laboratory. Of these five fractions, butanol fraction coded BFBu had a greater hypotensive effect compared to the other four fractions. BFBu was therefore chosen for this study. The extractions were repeated several times in order to obtain a sufficient amount of extract to perform the tests. BFBu was stored at -5 °C in a tightly closed jar to prevent spoilage.
Methods of the Study of Subacute Toxicity
Preliminary Tests : The results of the acute oral toxicity study were used as the basis for the selection of the doses of BFBu that were administered to the rats. The initial dose level, selected on the basis of this orientation study, is well below the Maximum Tolerated Dose (MTD). At the end of these tests, the doses of 50; 500 and 1000 mg/kg bw were chosen to be the doses to use during this study.
Distribution and Treatment of Rats : Animals were divided into 4 homogeneous batches of 6 rats. This homogeneity of the different batches is a function of the body weight of the rats. The tests were carried out on a control batch and 3 batches of treated animals. According to the method described by [15], each rat from the control group received by gavage distilled water at 10 mL/ kg bw. Butanolic fraction (BFBu), diluted in distilled water was administered, by gavage, to each of the rats of the 3 test groups, respectively at doses of 50; 500 and 1000 mg/kg bw. Volume of FBu administered to the rats of each batch was less than 2 mL. Rats were given distilled water or BFBu daily by gavage every morning between 7 and 8 hours AM. GMT for 28 days.
Blood Sample : Days 7, 14, 21 and 28 of treatment, blood samples were taken on an empty stomach from the rats previously anesthetized with ether for 2 to 3 minutes, by puncture at the level of the orbital sinus of the eye, at the level of the eye using pastor pipettes according to the technique described by [16-18]. Thus, 2 to 4 mL of blood from each animal of the 4 batches were collected in dry tubes.
Determination of Biochemical Parameters : Automatic device (KENZA MAX Biochemis Try, France) was used for determination of the biochemical parameters. Blood collected in dry tubes was centrifuged at 3000 rpm for 10 min and the resulting serum was stored at -20 °C until assaying for biochemical blood parameters. Creatinine, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed by cinetic method [19]. Urea was determined by enzymatic method [19,20]. HDL (High Density Lipoprotein) Cholesterol Assay Method in VITROS HDL Plate is based on a non-HDL cholesterol precipitation technique followed by enzymatic detection [21]. Measurements of serum concentrations of total proteins, total cholesterol, triglycerides, bilirubins (direct and indirect) were carried out by the colorimetric method [19-23]. Glucose present in the serum was determined according to the glucose oxidase colorimetric method with the “Glucose Trinder GPO-POD” kit [20,24]. Finally, the electrolytes (magnesium, calcium, sodium, potassium and chlorine) were also determined by the colorimetric method [25-28].
Evaluation of the Atherogenicity Index
Atherogenicity index of rats was determined according to the formula of [29].
Atherogenicity index = total cholesterol / HDL − cholesterol
Statistical Analysis
Statistical analysis of data and graphical representations were performed using Graph Pad Prism 5.01 software (San Diego, USA). The results obtained were expressed as the mean followed by the standard error of the mean (M±ESM). Difference between the mean values of the biochemical parameters was determined by one-way analysis of variance (ANOVA 1) and supplemented by Tukey’s test for the comparison of the means of the parameters of the different test groups compared to the control group. The significance level was set at p<0.05.
Effects of Bfbu on Biochemical Parameters
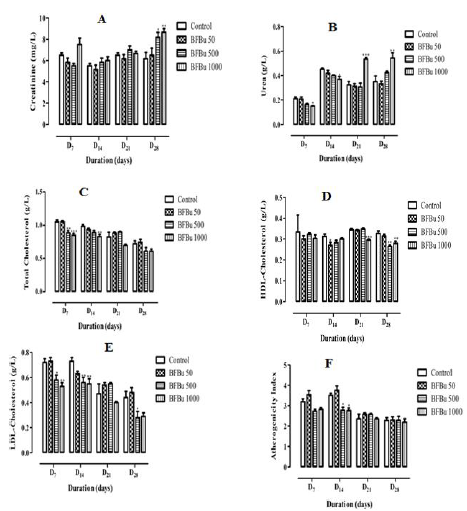
Effect of Bfbu on Creatinine Level : The mean creatininemie of the treated batch with BFBu at 50 mg/kg bw is lower than that of the control from D7 to D21 with the exception of D28 where it is higher than that of the control. D7, the creatinine level of the treated batch with BFBu at 500 mg/kg bw is lower than that of the control. However, from D14, this rate increases until the end of this study with a significantly (p<0.05) high rate with a value of 8.17±0.48 mg/L on D28. Only the creatinine level of the batch treated with BFBu at 1000 mg/kg bw increased throughout this study. This increase of 8.67±0.33 mg/L is significant (p<0.01) on the 28th day of treatment compared to the creatinine level of the control batch which is 6.17±0.60 mg/L (Figure 1A).
Effect of Bfbu on the Urea Rate: In the treated batches with BFBu at 50 and 500 mg/kg bw, the urea levels decreased compared to the control during this study, with the exception of D28 where there was an increase in this level. However, this decrease remains insignificant throughout this study. Moreover, from D7 to D14, in the treated batch with BFBu at 1000 mg/kg bw, a significant drop (p<0.05) in the urea level was noted compared to the control. Rate decreases and passes respectively from 0.21±0.02 g/L to 0.15±0.0 g/L and from 0.45±0.01 g/L to 0.37±0.02 g/L. However, from the 21st day of treatment, the amount of urea increased significantly (p<0.01-0.001) in this batch compared to the control batch. The urea rate increases and goes from 0.33±0.03 g/L to 0.54±0.01 g/L on D21 and from 0.35±0.04 g/L at 0.54±0.04 g/L on D28 (Figure 1B).
Effect of Bfbu on Total Cholesterol Level: Total cholesterol level of the treated batch with BFBu at 50 mg/kg body weight is lower than that of the control on D7 and D14. However, from D21, this rate undergoes a non-significant increase compared to the control. Moreover, the treated batches with BFBu at 500 and 1000 mg/kg bw, the total cholesterol level decreased during this study with the exception of the treated batch with BFBu at 500 mg/kg bw where this rate increased on D21. This decrease in mean cholesterolemia is significant (p<0.01-0.001) on D7 and D14 respectively compared to the mean value of the cholesterol level in the control batch, but it normalizes from D21. The cholesterol level decreases from 1.05±0.03 g/L to 0.85±0.03 g/L on D7 and from 0.99±0.03 g/L to 0.83±0, 03 g/L on D14 (Figure 1C).
Effect of Bfbu on Hdl-Cholesterol Levels: The mean HDL cholesterol values of the batch of rats treated with BFBu at 50 mg/ kg bw remained lower than that of the control during this study with a significant decrease (p<0.05) from a value of 0.27±0.01 g/L on D14. As for the HDL cholesterol level in the batch of rats treated with BFBu at 500 mg/kg bw, a decrease was noted during this work with the exception of D21 where its mean value is similar to that of the control. The mean value drops significantly (p<0.01) from 0.33±0.01 g/L to 0.27±0.01 g/L on D28 at this dose. Finally, in the batch treated with BFBu at 1000 mg/kg bw, the level of HDL cholesterol decreased throughout this study with a significant drop (p<0.001-0.01) in this value which went from 0.35±0.01 g/L at 0.30±0.00 g/L and from 0.33±0.01 g/L to 0.28±0.01 g/L respectively on D21 and D28 after administration of BFBu (Figure 1D).
Effect of BFBu on LDL-Cholesterol Levels: In the treated batch with BFBu at 50 mg/kg bw, the LDL-cholesterol level did not undergo any significant variation from D7 to D28 compared to that of the control batch In the treated batches with BFBu at 500 and 1000 mg/kg bw, the level of LDL-cholesterol decreased during this study. This drop is significant (p<0.05-0.01) on D7 and D14 in these two batches. From D21, BFBu does not cause any significant variation in the LDL cholesterol level with the exception of the treated batch with FBu at 500 mg/kg of bw where a significant drop (p<0.05) in this level was recorded on D28 with a value of 0.28±0.05 g/L (Figure 1E).
Evaluation of the Atherogenicity Index of BFBu: At a dose of 50 mg/kg bw, BFBu did not cause any significant increase in the atherogenicity index compared to that of the control group during this study. In the treated batch with BFBu at 500 mg/kg bw, there was a non-significant decrease on D7 followed by a significant decrease (p<0.05) in this index on D14 with a value of 2.77±0.16. From D21 to D28, the atherogenicity index of this batch remains similar to that of the control batch. Finally, in the treated batch with BFBu at 1000 mg/kg bw, the atherogenicity index remained low throughout this study compared to the mean value of the control batch. This drop is significant (p<0.05) on D14 where the mean value is 2.76±0.12 (Figure 1F).
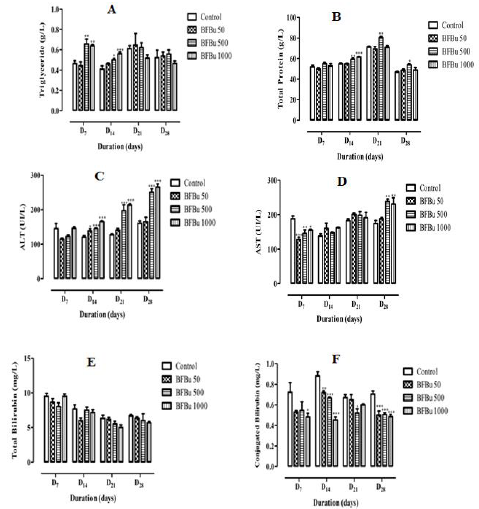
Effect of BFBu on Triglyceride Levels: Repeated administration of BFBu at 50 mg/kg bw causes a non-significant decrease in the triglyceride level on D7 followed by an increase in this level from D14 until the end of this study. In the presence of BFBu at 500 mg/kg bw, the triglyceride level increases during this study with a significant increase (p<0.01-0.05) respectively on the 7th and 14th day of treatment. The respective average values recorded are 0.66±0.05 g/L and 0.50±0.01 g/L. In the treated batch with BFBu at 1000 mg/kg bw, a significant increase (p<0.01-0.001) in the level of triglycerides was noted respectively on D7 and D14 where the average levels are 0.64±0.01 g/L and 0.56±0.02 g/L. This increase is followed by a non-significant drop in triglyceridemia compared to the control batch on D21 and D28 (Figure 2A).
Effect of BFBu on Total Protein Level: Total protein level of the treated batch with BFBu at 50 mg/kg bw remained almost lower than that of the control during this study with the exception of D28 where this rate increased from 46.80±0.95 g/L at 48.70±1.05 g/L. Variations caused by BFBu at this dose are not significant (p≥0.05) compared to the control batch. In the treated batches with BFBu at 500 and 1000 mg/kg bw, an increase in this rate was noted throughout this work. This increase is significant (p<0.05) in the treated batch with BFBu at 500 mg/kg bw from D14. The increase in total protein level caused by BFBu at 1000 mg/kg bw is only significant (p<0.001) on the 14th day of treatment where its value is 61.50±0.43 g/L (Figure 2B).
Effect of BFBu on Alanine Aminotransferase Level: Alanine aminotransferase (ALT) level of the treated batches with BFBu at 50 and 500 mg/kg bw is lower than that of the control batch on D7. However, from D14 until the end of this study, the level of ALT increased in these batches compared to that of the control batch. The increase in this level in the treated batch with BFBu at 500 mg/kg bw is significant (p<0.001) from this period, whereas the increase in this rate is only exceptionally so on the 14th day in the lot treated with BFBu at 50 mg/kg bw where its value is 138±6.63 IU/L. In the treated batch with BFBu at 1000 mg/kg bw, the level of ALT, which remained almost the same to the control on D7, underwent a significant increase (p<0.001) from D14 to D28 with values of 165±2.63 IU/L (J14), 213±3.62 IU/L (J21) and 265±9.56 IU/L (J28) (Figure 2C).
Effect of BFBu on Aspartate Aminotransferase Level: On D7, all the doses of BFBu tested caused a significant decrease (p<0.05- 0.001) in the level of aspartate aminotransferase (AST) compared to the control. The mean AST value of the control batch is 188±7.92 IU/L. The AST levels recorded are 128±6.94 IU/L, 145±11.00 IU/L and 155±2.79 IU/L respectively for doses of BFBu of 50, 500 and 1000 mg/kg bw. However, from D14, an increase in the AST rate was recorded in all the batches treated with BFBu. This increase was not significant in all of the batch test from D14 to D21. On D28, in the treated batches with BFBu at 500 and 1000 mg/kg bw, a significant increase (p<0.01) in its mean value was recorded compared to that of the control batch (Figure 2D).
Effect of BFBu on Total Bilirubin Level: All tested doses of BFBu, the mean values of the total bilirubin level underwent a nonsignificant decrease (p≥0.05) compared to that of the control lot during this study with the exception of the lot treated with BFBu at 1000 mg/kg bw on D7 where this rate, a value of 9.50±0.34 mg/L, remained almost identical to that of the control (Figure 2E).
Effect of BFBu on Conjugated Bilirubin Level: With regard to the level of conjugated bilirubin, the mean values in the presence of all the doses of BFBu tested are lower than that of the control during this study. At 50 mg/kg bw, a significant drop (p<0.01-0.001) in the conjugated bilirubin level was recorded on D14 and D28, respectively, but this decrease was not significant on D7 and D21. At 500 mg/kg bw, the conjugated bilirubin level underwent a significant decrease (p<0.05-0.001) from the 14th day of treatment with the exception of D7 where this decrease is not significant with a value of 0.55±0.08 mg/L. Finally, at 1000 mg/kg bw, the level of conjugated bilirubin decreases significantly (p <0.05 - 0.001) during the treatment except on D21 when a non-significant drop in its value compared to the control was recorded. (Figure 2F).
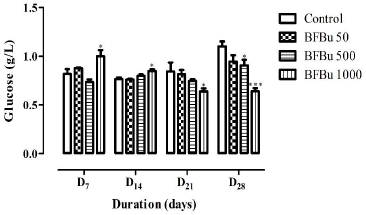
Effect of BFBu on Glucose Level: No significant variation in blood glucose was recorded in the treated batches with BFBu at 50 and 500 mg/kg bw compared to the control during this study. On the 7th and 14th day of treatment, the mean values of the blood glucose level underwent a significant increase (p<0.05) in the treated batch with BFBu at 1000 mg/kg bw compared to the control batch. The average blood sugar values are 1.00±0.06 g/L (D7) and 0.85±0.02 g/L (D14). However, from the 21st to the 28th day of treatment, a significant decrease (p<0.05-0.001) in the blood glucose of the rats was observed in the treated batch with FBu at 1000 mg/kg bw compared to the mean value of the glycemia of the rats of the control group (Figure 3).
Effects of BFBu on Electrolytes
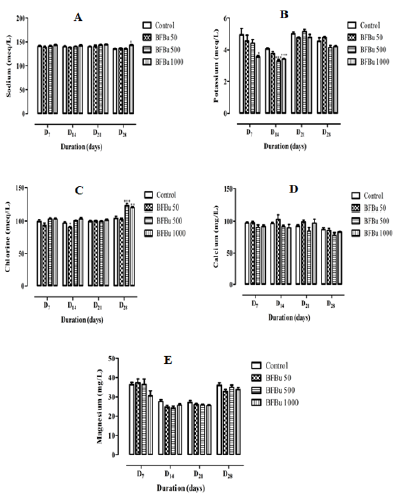
Effect of BFBu on Sodium Level: Repeated administration of BFBu at doses of 50, 500 and 1000 mg/kg bw did not impacted with sodium levels during this study with the exception of the batch treated with BFBu at 1000 mg/kg bw where an increase significant (p<0.05) was recorded on the 28th day of treatment compared to the control batch. The sodium level is 143±1.31 meq/L at this dose (Figure 4A).
Effect of BFBu on Potassium Level: At 50 mg/kg bw, BFBu induces a drop in the potassium level with the exception of D28 where this rate increased compared to the control. These variations are not significant at this dose. At a dose of 500 mg/kg bw, the potassium level is lower than that of the control during this study. The drop in this rate is significant (p<0.001) on the 14th day of treatment in this batch where a value of 3.30±0.10 meq/L was recorded. However, on D21, the potassium level is higher than that of the control. As for the treated batch with BFBu at 1000 mg/kg bw, the potassium level remained lower than that of the control during this study with significant decreases (p<0.05-0.001) noted respectively on D7 and D14 but not significant of D21 to D28 compared to the control batch (Figure 4B).
Effect of BFBu on Chlorine Level: Chlorine level of the treated batch with BFBu at 50 mg/kg bw decreased significantly (p<0.05) on D14 compared to the control batch. As for the treated batches with BFBu at 500 and 1000 mg/kg bw, the chlorine level increased during this study with exceptionally significant increases (p<0.01-0.001) in this rate on D28 compared to the control batch. The respective values recorded at these doses are 122±3.53 meq/L and 119±1.50 meq/L. However, on D21, the chlorine level is almost identical in all the batches treated with BFBu (Figure 4C). Effect of BFBu on Calcium Levels: The calcium level of the treated batches with BFBu at 50, 500 and 1000 mg/kg bw did not undergo any significant variation (p≥0.05) during this study compared to the control (Figure 4D).
Effect of BFBu on the Magnesium Level: On D7, the magnesium level of the treated batches with BFBu at 50 and 500 mg/kg bw is higher than that of the control. However, from the 14th day of treatment, this rate decreased in these batches compared to the control. Only the magnesium level of the treated batch with FBu at 1000 mg/kg bw continuously decreased during this work. All the variations caused by BFBu at the doses tested remained insignificant (p≥0.05) compared to the control during this study (Figure 4E).
The various biochemical parameters examined in this study are useful indicators for assessing the toxicity of plant extracts in animals [30]. Analysis of blood parameters is relevant in risk assessment since changes in the hematologic system have a very high predictive value for toxicity in humans [31]. The significant reduction in the level of transaminases (AST and ALT) observed at the start of the experiments indicates that the absorption of BFBu does not cause cytolysis but rather a protective effect in the liver. BFBu does not harm the liver within the first week of administration. However, at the end of this study, the increase in these parameters following the administration of BFBu could be due to the appearance of necrosis in one or more organs, namely the liver, heart and kidneys, thus causing an increase in the level of these enzymes in the blood. Indeed, according to [32], damaged tissue is generally associated with the release of specific enzymes from the tissue, or the affected organ, into the bloodstream. The consequence is an increase in the activity of such enzymes in bodily secretions. ALT is a cytoplasmic enzyme found in very high concentrations in the liver, and an increase in this specific enzyme suggests liver cell damage while AST is less specific than ALT as an indicator of liver function. The increase in the level of transaminases (ALT and AST) has also been observed in liver disorder [33-36]. These results are in agreement with those of [37,38] who reported that the ethanolic and methanolic extracts of Alchornia cordifolia leaves cause an increase in the activity of transaminases. These authors therefore suggested that these extracts exhibit hepatotoxic effects at doses of 250 to 500 mg/kg bw and 800 to 1600 mg/kg bw, respectively. BFBu would therefore be, at the tested doses of 500 and 1000 mg/ kg bw and in the long term, probably poorly tolerated by the liver. According to [39], the increase in the serum total protein level is an indication of tissue damage while the significant decrease in the total protein content of the liver is a reflection of hepatic nontoxicity. The significant increase in the total protein content in the batches of rats treated with BFBu at 500 and 1000 mg/kg bw could therefore indicate an increase in protein stores and therefore suggest hepatic toxicity, which would again confirm the detrimental effects of BFBu at 500 and 1000 mg/kg bw. Bilirubin, a metabolic breakdown product of heme derived from senescent red blood cells, is also one of the most commonly used tests for liver function. Its concentration could indicate the condition of the liver and the type of liver damage [40,41]. The significant reduction in total and conjugated bilirubin levels could be provided by a deficiency in the secretory function of these proteins. Certain substances present in FBu could therefore imply inhibitions at the level of the hepatic cells. This reduction can also affect the functional activity of the liver. Doses of BFBu of 50 and 500 mg/kg bw did not significantly affect blood sugar. Only the dose of 1000 mg/kg bw increased it on D7 and D14 and decreased on D21 and D28. These results suggest that this fraction has hyperglycaemic activity followed by hypoglycaemic activity at high doses. At the end of this study, the hypoglycaemic activity could be attributed to the existence of the molecules demonstrated in this fraction. Indeed, flavonoids from different plants have shown a promising hypoglycemic effect in diabetic animal models [42-44]. Saponins are triterpene, steroidal or alkaloidic glycosides. Authors have shown a hypoglycemic activity of triterpene glycosides [45,46]. It is therefore possible that the presence of flavonoids and saponins in FBu could be responsible for the hypoglycemic effects of this fraction. Some authors reported a significant decrease (p<0.05) in the fasting blood glucose level of rats made diabetic with alloxane when given the ethanolic extract of Ficus microcarpa, orally for two weeks [47]. Finally, these results are in agreement with the studies conducted respectively on the seed cotyledon and leaves of Chrysophyllum albidum at doses between 250 and 1000 mg/kg bw [48,49] on the one hand, and on the other hand on Bixin (10 mg/kg bw) extracted from the seeds of Bixa orellana [50]. These authors have shown that these extracts significantly decrease the glucose level in normal and diabetic rats induced by alloxane. However, the notable increase in blood sugar observed in this study could be linked mainly to the stress undergone by the animals at the time of sampling as suggested by [51] during work with Momordica charantia in rabbits. However, a real hypoglycemic effect could be attributed to this fraction by carrying out further study on suitable models. The significant (p<0.01) increase in mean serum creatinine was seen in the lots that received the highest doses of FBu (500 and 1000 mg/kg bw). This result suggests that BFBu is toxic to the kidneys. Creatinine is the major catabolic product of muscles and is secreted by the kidneys. Serum creatinine levels are used as an indicator of kidney defects [38,52,53]. The significant drop in the urea level observed at the start of treatment in the batch treated with FBu at 1000 mg/kg bw would be a sign of the good functioning of the kidney and the lack of renal toxicity due to this fraction. However, the increase in this level in this batch would be an indication of azotemia. According to Nduka [54], the high urea level is associated with the increased catabolism of tissue proteins. The excess protein in the blood collapses and urea excretion decreases, increasing its level in the blood. The increase in this level would also be a sign of damage to the kidney, which can no longer extract nitrogenous excretion products from the blood and concentrate them in the urine, thus confirming the renal toxicity caused by BFBu. Other indicators of kidney function such as sodium, potassium and chlorine were significantly affected by BFBu except calcium and magnesium which were not. The urea, sodium and chlorine levels significantly increased in the treated batches with BFBu at the highest doses during this study. These results probably suggest nephrotoxicity of BFBu at high doses, which would support the hypothesis made about the increased creatinine level. However, the lack of a significant effect of BFBu on serum calcium and magnesium concentrations in animals suggests that the secretory capacity of the kidney and normal organ function related to these parameters were not affected. According to the work of [41] on the aqueous extract of Felicia muricata at doses of 50, 100 and 200 mg/kg bw, the calcium and magnesium levels of the rats were not significantly modified. The hypokalaemia caused by repeated administration of BFBu may be due to an alteration in tubular reabsorption of potassium. Similar effects have been reported by [55] with the aqueous extract of Arctotis arctotoides on the decrease in potassium concentration. These authors suggested that this plant would not cause any damage to the cardiovascular system, which could also be the case with BFBu. Changes in the concentration of major lipids such as total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides, as well as the atherogenicity index may provide useful information on lipid metabolism and heart predisposition, atherosclerosis and its associated coronary heart disease [29,56-58]. In this study, the decrease in serum cholesterol level could be explained by the deterioration of cholesterol biosynthesis. This has also been suggested by [59] who showed that the aqueous extract of Chrysocoma ciliata at doses of 50, 100 and 200 mg/kg body weight decreases the cholesterol level in rats. Elevated cholesterol concentration is an important risk factor for cardiovascular disease [60]. Therefore, lowering cholesterol at the doses of BFBu tested could be clinically beneficial as this fraction is unlikely to be associated with cardiovascular risk. Triglycerides are the storage forms of fatty acids. The decrease in the level of lipids such as HDL and LDL cholesterols as well as the decrease in the atherogenicity index may be an indication that FBu would not predispose animals to the risk of atherosclerosis and associated coronary heart disease. According to several authors, the total cholesterol/cholesterol- HDL ratio constitutes a revealing index of arterial and especially coronary risk. This ratio would indicate an increased risk if it is greater than 4.4 [29]. However, according to the results obtained during this study, the atherogenicity index of each animal was lower than this value. BFBu could therefore be protective of heart tissue. Furthermore, the decrease in serum lipid parameters investigated in this study suggests that the lipid metabolism of animals was disturbed by the administration of BFBu. This disturbance could be an indication that BFBu would probably not predispose the animal to atherosclerosis and associated coronary heart disease despite the notable elevation (p<0.001) in triglyceride levels during the first 14 days in batches treated with BFBu at 500 and 1000 mg/ kg bw.
This study showed that butanol fraction of Blighia unijugata (BFBu) produced changes in biochemical parameters following repeated administration. BFBu caused disruption of the carbohydrate, lipid and protein balances. At high doses, BFBu caused hepatic and renal toxicity. However, the fraction tested at dose of 50 mg/kg bw, is non-toxic. This fraction has hypoglycemic effects and could be used in the management of diabetes, respectively. BFBu would not predispose rats to the risk of atherosclerosis and associated coronary heart disease.
For more Articles on: https://biomedres01.blogspot.com/