An Additional, Complementary Mechanism of Action for Folic Acid in the Treatment of Megaloblastic Anemia
Abstract
Administration of supplemental folic acid addresses the impaired DNA
synthesis causing megaloblastic anemia; however, despite the possibility
of high doses of folic acid -5 to 15 milligrams (mg) daily for up to
four months -the extremely rapid initial onset of action -30 to 60
minutes -when administered orally is not in keeping with the accepted
mechanism, which can take up to nearly 22 hours, even under enzymatic
control. This would suggest an additional, complementary non-enzymatic
mechanism of nucleotide methylation at work; it is, therefore, proposed
here, with in vitro evidence put forth, that rapid, non-enzymatic
methylation by folic acid, via 5,10-methylenetetrahydrofolate and
7,8-tetrahydrofolate intermediates, of deoxyuridine monophosphate to
form thymidylate using the cell membrane phospholipid
phosphatidylcholine as a methyl donor, leaving de-methylated
phosphatidylethanolamine, is a viable additional and complementary
mechanism in helping reduce megaloblastic red blood cells to normal size
and function.
Keywords: Folic Acid; DNA; Megaloblastic Anemia; Deoxyuridine Monophosphate; Thymidylate
Abbreviations: PC: Phosphatidylcholine;
DUMP: Deoxyuridine 5′-Monophosphate; DHFR: Dihydrofolate Reductase; THF:
Tetrahydrofolate; dTMP: Deoxythymidine 5′-Monophosphate; SAM-e:
S-Adenosylmethionine; PE: Phosphatidylethanolamine; TMS: Trimethylsilyl;
PCCA: Professional Compounding Centers of America; NS: Normal Saline;
HCL: Hydrochloric Acid
Introduction
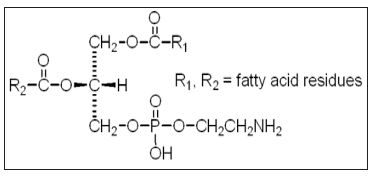
Dietary deficiency of the vitamin enzyme cofactor folic acid (Figure
1) has serious effects upon human health, including chromosome breaks,
birth defects such as neural tube defects, increased risk of colon
cancer, brain dysfunction, and heart disease [1]. Its deficiency is also
the cause of megaloblastic anemia, or megaloblastosis, an anemic blood
disorder characterized by larger-than-normal red blood cells (RBCs), or
megaloblasts [2]. Two hallmarks of megaloblasts are, first, elevated
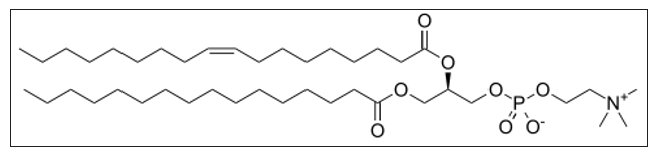
levels of the cell membrane phospholipid phosphatidylcholine (PC) [3]
(Figure 2)
and, second, increased amounts of the nucleotide deoxyuridine
5′-monophosphate (dUMP), which is otherwise usually methylated by the
folic acid derivatives 5,10-methylenetetrahydrofolate and
7,8-dihydrofolate, via the thymidylate synthase enzyme pathway, which
also includes the enzyme dihydrofolate reductase (DHFR), the coenzyme
nicotinamide adenine dinucleotide in both its reduced and oxidized forms
(NADH and NAD+, respectively), and the folic acid derivative
tetrahydrofolate (THF), to deoxythymidine 5′-monophosphate (dTMP), or
thymidylate, as part of normal deoxyribonucleic acid (DNA) synthesis [4]
(Figure 3).
Figure 2: Phosphatidylcholine (PC).

Administration of supplemental folic acid addresses the impaired
DNA synthesis causing megaloblastosis; however, despite
the possibility of high doses of folic acid - 5 to 15 milligrams (mg)
daily for up to four months - the extremely rapid initial onset of
action - 30 to 60 minutes - when administered orally [5] is not in
keeping with this accepted mechanism, which can take up to nearly
22 hours, even under enzymatic control [6]. This would suggest an
additional, complementary non-enzymatic mechanism of nucleotide
methylation at work. Such non-enzymatic methylation of nucleotides
has been observed previously in vitro with the metabolite
cofactor S-adenosylmethionine (SAM-e) [7]; it is, therefore, proposed
here, with in vitro evidence put forth, that rapid, non-enzymatic
methylation by folic acid, via 5,10-methylenetetrahydofolate
and 7,8-dihydrofolate intermediates, of dUMP to form thymidylate
using PC as a methyl donor, leaving the de-methylated cell membrane
phospholipid phosphatidylethanolamine (PE) (Figure 4), is a
viable additional and complementary mechanism in helping reduce
megalobastic RBCs to normal size and function.
Experimental
All research was conducted at The Wellness Pharmacy in
Winchester, VA, USA from February of 2018 through March of 2018.
All sterile manipulations were performed using aseptic technique
[8] in a NUAIRE Biological Safety Cabinet Class II Type A/B3 laminar
flow hood. All pH measurements were made using a Horiba TwinpH
waterproof B-213 Compact pH Meter. All Gas Chromatography/
Mass Spectrometry (GC/MS) data was obtained using a Hewlett
Packard 5890 Series II Gas Chromatograph. Compounds were
identified as the trimethylsilyl (TMS) derivatives by using the
agreement of the retention times with those of standards as the
criterion for identification and the following ions for determination:
m/z 321.0488 for thymidylate [9], and m/z 255.13 for PE [10].
All in-laboratory photography was obtained using a Casio EX-Z57
digital camera. All Pyrex glassware was sterilized at 130° C for one
hour [11] via autoclave using a Quincy Lab Inc. Model 30 GC Lab
Oven. All chemical supplies were purchased from the Professional
Compounding Centers of America (PCCA) in Houston, TX, USA.
All measurements of chemicals were standardized to 0.1
Molarity (M)5% using an Ohaus Analytical Plus electronic
balance accurate to within 0.0001 gram (g). Final test and control
samples were obtained via filtration through a sterile 0.2 micron
(m) EPS, Inc. Medi-Dose Group Disposable Disc Filter Unit and
corresponding sterile Monoject syringe. Three trials per step were
performed and recorded and the data presented here represents the
average of that total data. All data collected fell within a statistically
acceptable 5% (p = 0.05) internal margin of variance [12] with
no outliers. As a control, 25 milliliters (mL) of sterile 0.9% sodium
chloride in water (normal saline (NS)), pH 7.4, was heated to 37 C°
and maintained, with 0.45 g of PC (95% ± 5%) added and allowed
to melt uniformly throughout (Figure 5). To this was added 0.015
g of folic acid (Figure 6) and the pH adjusted first downward to 2
via titration with 0.1 M hydrochloric acid (HCl), then upward to 10
via titration with 0.1 M sodium hydroxide (NaOH) solution. The
pH was then adjusted back to 7.4 via HCl. As a test, to a second,
similarly prepared mixture at pH 7.4 and 37 C°, 0.45 g of highly
solubilized dUMP (95% ± 5%) was added (Figure 7), subsequent
pH measured, and its contents compared to the first mixture
(Figure 8). Two samples were procured from the second mixture
for GC/MS analysis, one to test for the presence of thymidylate and
one to test for the presence of PE.
Figure 5: Control mixture of 25 mL NS, pH 7.4, at 37 C°
with 0.45 g PC (95% ± 5%) melted uniformly throughout.

Results and Discussion
At physiologic salinity, pH, and temperature [13], no
precipitation of any of the components of the control mixture
was observed; furthermore, no precipitation was observed in
this mixture as a function of pH within the stability range of a
therapeutically relevant quantity of folic acid [14] and PC [15].
Precipitation, however, was observed in the test mixture, beginning
immediately upon the addition of dUMP and reaching completion
within 10 minutes after its addition. The pH of the test mixture
was 6.2. Such precipitation, ruled out as a function of pH, indicated
an increase in hydrophobicity, as would be the case if hydrophilic
dUMP were being converted into more hydrophobic thymidylate
[16]. Also, the change in appearance of the mixture from opaque
to translucent indicated the conversion of PC into PE [17]. GC/
MS analysis confirmed the presence of both thymidylate (Figure
9) (95% ± 5%) and PE (Figure 10) (95% ± 5%). The mechanism
at work here appears to be extremely rapid and non-enzymatic in
nature.
It most likely involves successive de-methylation of PC
by folic acid to form, first, a 5,10-methylenetetrahydrofolate
intermediate and PE, with the former then donating its methylene
group to methylate dUMP into thymidylate, leaving a second,
7,8-dihydrofolate intermediate, which then quickly oxidizes back to
folic acid. Such a supposition is supported by observations of these
two intermediates’ formation by transient, acidic changes in pH by
De Brouwer et al [18]. This transient nature, however, along with
the extremely rapid rate of reaction observed in this experiment,
precludes their detection here, although its inferred presence
seems to best account for the observed results. Such a rapid, nonenzymatic
mechanism would also account for the quick onset of
action of folic acid therapy in the treatment of megaloblastic anemia,
acting in an additional and complementary fashion with that in
already elucidated enzymatic mechanisms. It also establishes folic
acid, alongside SAM-e, as a powerful agent involved in the nonenzymatic
methylation of endogenous nucleotides.
Acknowledgement
I thank the staff of The Wellness Pharmacy for the generous use
of their facility and equipment. I also especially thank my loving
family for their generous gift of time in the performance of these
experiments and the writing of this article. This article is dedicated
to the memory of Raymond Burnell Knepp (1942-2009), founder of
The Wellness Pharmacy.
Fostering Recovery and Functional Engagement
of Children With Traumatic Brain Injury through
Technological Supports: A Mini Review - https://biomedres01.blogspot.com/2020/03/fostering-recovery-and-functional.html
More BJSTR Articles : https://biomedres01.blogspot.com






No comments:
Post a Comment
Note: Only a member of this blog may post a comment.