In Vitro Characterization of Mems Based Piezoelctrically Actuated Drug Delivery Device for Biomedical Applications
Abstract
Implantable devices that detect and treat diseases without any
intervention required from the patient are expected to be the trend of
the future. This paper presents a peizo electrically controlled MEMS
drug delivery device for on-demand release of defined quantities of drug
in a sustained and controlled manner. A drug-loaded polymer based micro
reservoir (600μm ×550μm) is sealed by a Polydimethylsiloxane (PDMS)
membrane placed over the drug reservoir on which the piezoelectric
material is deposited. On application of voltage across this
piezoelectric material, the membrane deflects allowing the fluid to fill
into the chamber that will mix with the drug and due to concentration
variation; the drug would come off the reservoir or vice versa. A
0.3μm-thick PZT material is deposited on 20μm PDMS membrane. Discharge
of the drug solution and the release rates were controlled by an
external electric field. Characterization of the devices was implemented
in-vitro using the colored water solution. The reservoir was capable of
delivering 20μl drug on application of 10V.
Keywords: Polydimethylsiloxan; Drug Delivery; PZT Materialy
Introduction
In recent years, one of the most exciting progresses in MEMS
application is the rapid evolution of Biological-Microelectromechanical
systems (BIOMEMS). The BIOMEMS has gained its attention due to the
microfabrication technology which has been applied to the successful
development of a variety of health care related products. The research
on microfabricated devices for medical application is gaining more
attention. The microfabricated drug delivery system and its utility in
the medical application have become a major topic of research [1-4]. In
addition to basic components, such as micro channels, microvalves,
micropumps, micro mixers and micro-reactors for flow management at
microscopic volumes, various novel sensor and detection platforms have
been reported in the micro-fluid and BIO-MEMS fields. Many of the
so-called micro total analysis systems (μ-TAS) or lab-on-a-chip systems
have also been reported and will offer new paradigms in biomedicine and
biology, in particular, the ability to perform point-of-care
measurements [5-6]. A microchip delivery system consists of a substrate
which consists of fabricated reservoirs capable of holding chemicals in
the solid, liquid, or gel form. The microfabrication of these devices
includes numerous techniques such as lithography, thin film deposition,
etching, and so on [7]. The Microfabrication technology for drug
delivery system
has many advantages over the traditional drug delivery approach which
uses spherical drug delivery principle [8]. The use of micro technology
offers a number of advantages which may modernize the field of
controlled release. Microfabrication also offers precise control over
shape, size, and geometry of delivery devices which in turn can increase
the drug loading capacities and provide better control over drug
release. Single microfabricated devices can integrate multiple
reservoirs with different drug or bio-molecules and can be filled in
with Pico to nanoliters of the solution, this offers a significant
advantage of releasing the drug in a multi-directional way which makes
it unique from the unidirectional spherical drug delivery system [9-11].
There is extensive work being carried out to use Polydimethylsiloxane
(PDMS) which is considered to be bio compatible, less expensive as the
main material for different drug delivery systems for cancer treatment,
HIV treatment etc. PDMS based drug delivery systems can be used to
achieve prolonged release profiles [12]. The drug that will be loaded
into the PDMS structures can be sustained for months. The drug delivery
is controlled by different actuation techniques such as diffusion or
magnetic actuation [13-15]. The developed device is capable of
delivering a required quantity of drug to the targeted site using
piezoelectric actuation technique. The device was actuated using
piezoelectric actuation technique. This non-invasive fabricated
device provides reusability, precise control and can enable the
patient or a physician to actively administrate the drug as and when
required.
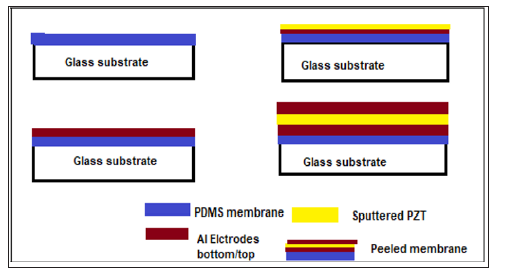
Device Fabrication
The molds for the micro reservoirs, were made using
photolithography by SU-8 2150; (MicroChem Corp., MA, USA)
negative photoresist was spin-coated on a glass substrate and
patterned. Cured PDMS [Sylgard 184 Silicone Elastomer, Dow
Corning Corp oration] was then molded using the SU8 pattern
(Figure 1). Also a 20μm thick PDMS membrane was prepared by
spin coating the PDMS on the glass substrate. These reservoirs and
the membrane are then treated with argon-induced plasma for
15mins and test liquid was filled in the reservoir as a sample drug.
The treated membrane was then metalized (aluminium) followed
by the sputtering of 0.3μm PZT layer and later evaporated for
aluminium electrode using masks. The membrane was irreversibly
bonded to the reservoir layer. Next, an aperture of 100*100μm
2 was drilled with a UV laser (254 nm wavelength). The bellow
diagram describes the flow of the fabrication of PZT-Electrodes.
Results and Discussion
Unlike the conventional drug delivery devices, the fabricated
Piezoelctrically actuated device can be used to deliver the drug to a
specific target [16-18]. The PDMS membrane and the reservoir are
made hydrophilic by argon plasma treatment, in order to prevent
the adsorption of the drug on the PDMS surface. This surface
modification is also required to have a uniform deposition of the
PZT material. The fabricated device is shown in Figure 2. The device
was fabricated with different membrane thickness with 5μm, 10μm
and 20μm thickness. The actuation test was also carried out varying
the deposition thickness of PZT for different membrane thickness.
It was observed that increase in PZT thickness with constant
membrane thickness results in less voltage application which is
an advantage of this present device. The table shown in the below
Table 1 summarizes the voltage required to actuate the device with
different thickness.
Actuation Test
The actuation of the PZT membrane was achieved by applying
variable voltages across the PZT electrodes. The device with test
liquid was immersed in water. The voltage across the electrodes
was slowly increased in steps. It was observed that there was
permeation of the test liquid with respect to the increased voltage.
it was further proven using the microscopic testing of the obtained
sample with changed colour. The release of the drug is due to
the deflection of the PZT membrane. The rate of drug flow was
controlled by the actuation voltage. The direction of deflection of
the membrane can be controlled by the direction in which electric
field is applied across the electrodes. The graph in Figure 3 shows
the flow of drug Vs the applied voltage. The maximum amount
of the test liquid delivered was found to be 20μL. The volume of
drug loaded can be varied by changing the size of the reservoirs.
The purpose of this drug delivery device was to measure the
drug release as a function of external actuation and not for the
characterization of the drug. The percentage of cumulative drug
release was calculated considering the device of PZT thickness
0.5μm and it was calculated by the below equation
(%) = × 100
Conclusion
Advances in drug delivery systems have brought better control
rates and increased efficacy. The developed DDS can be used for
both the liquid and solid drugs. The device finds many applications
where targeted and controlled delivery is required, such as
protein delivery, insulin delivery, chemotherapy etc. The actuation
method of the device was verified along with all the unit steps
and it is concluded that increasing voltage across PZT increases
the deflection of the membrane which ultimately will be used to
deliver the drug into the target source. Different PZT materials with
differed properties such as relative permittivity, density, can be
used as electrodes for the actuation.
Titanium Plate Cranioplasty Induced Intravascular
Papillary Endothelial Tumor: Case Report
and Review of the Literature - https://biomedres01.blogspot.com/2020/03/titanium-plate-cranioplasty-induced.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.