An Analog of PKC412 Blocks Human Leukemia HEL Cells Growth Through STAT3/Mir-17-92/BIM Signal Pathway
Introduction
In the past few decades, cancer ranks as the most severe disease through uncontrolled cell growth, and it is a leading factor in death worldwide [1]. It is predicted that cancer deaths worldwide will reach more than 11.4 million [2,3]. So, the quantity demanded of anti-cancer drugs is sharp increasing all over the world. Sales of anti-cancer drugs have reached 36.4 billion US dollars and ranking the first among all kinds of medicines since 2010 [4]. Therefore, the prevalence of cancer and their resistance to the existing therapeutic agents necessitate the development of new medication that may overcome the limitation of existing drugs [5]. Leukemogenesis is induced through the emergence of neoplastic progenitors during hematopoiesis that subsequently undergoes clonal expansion leading to full- blown leukemia [6]. Leukemia contains four central subgroups: Chronic Myeloid Leukemia (CML), Acute Lymphoblastic Leukemia (ALL), and Chronic Lymphocytic Leukemia (CLL), Acute Myeloid Leukemia (AML) [7]. Midostaurin is the only FLT3 inhibitor approved by the FDA for the treatment of newly diagnosed FLT3-mutation AML in combination with systemic chemotherapy [8] and demonstrated antiproliferative activity in a range of solid tumor lines, including lung, colon, breast, melanoma, and glioblastoma [9]. Drug resistance has been the limiting factors in the success of available treatments for cancers, development of resistance to PKC412A has been described recently [10-12]. Here we synthesized an analog of midostaurin (PKC412), GZWM-060. Employing the cytotoxicity assay, we discovered that GZWM- 060 had potent activity against leukemia cells, with minimal IC50 value. Therefore, we determine the mechanism of anti-cancer activity furtherly and found that the compound could exert higher effective against HEL cells growth than PKC412.
Material and Method
Reagents
In a literature review we found 7 single case reports of psychosis secondary to clomiphene therapy in female (Table 2). Many (5 of 7) case repots have a known psychiatric history; but 2 cases do not (Table 2). In these 2 cases, it is likely that a remote preceding episode of subclinical psychiatric issue may have been unreported. For many patients, a former history of mental illness (e.g. bipolar affective disorder and depression) can be subtle if not exhaustively explored, such as the history of transient neurological dysfunction [3] or preceding suicide attempt and lifetime psychotic instability [4]. Grimm and Hubrich [5] describe the case with paranoid delusion even one day after the beginning of clomiphene therapy. In all cases, the temporal association of both the onset and resolution of their symptoms with clomiphene initiation (or during treatment) and discontinuance, respectively, it is plausible to conclude that the development of psychiatric symptoms results from clomiphene treatment.
Cell Culture
The human erythroleukemia cell line HEL was held by our laboratory. The cells were cultured in RPMI 1640 medium supplemented with 5% FBS at 37°C in a CO2 incubator (5% CO2 and 95% air, 95% humidity). The cells were passaged twice weekly to maintain an exponential growth phase.
MTT Assay
The cytotoxicity assay was accomplished by MTT assay. The assay is based on the alteration or conversion of MTT dye into red formazan derivatives. Cell plated at the density of 8000 cells per well in a 96 well-plate and treated with GZWM-060 at different concentrations (0.15 to 1 µmol/L). In cell cytotoxicity assays, control groups were treated with either 1% DMSO without GZWM-060. After 72 hours of treatment, per well were added MTT solution 20 µl (5mg/mL) for 4 hours respectively. 96 well - plate were centrifuged in 2500 rpm with 15min, and discard the medium, then DMSO (150 µl) was used to dissolve formazan crystals. The resulting solution was determined using absorbance at 490 nm (Thermo Scientific, Vario Skan Flash, USA). The growth curve of MTT was performed in 24, 48 and 72h. All experiments were carried out in triplicate and three independent tests. The percentage of cytotoxic activity compared to that of an untreated cell was determined as follows:
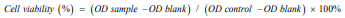
Apoptosis Assay
Cell were seeded at 1×10 5 cells per 60 mm dish in 3 mL medium with different concentration of GZWM-060 (0.15 to 1 µmol/L ), after grown 24, 48, 72h, the cell were harvested, washed 3 times with ice-cold PBS and transferred into microcentrifuge tubes for centrifugation at 220g for 5min at room temperature, then re-suspended in Annexin-binding buffer and 5µL of FITC and PI (BD FITC Annexin V Apoptosis Detection Kit I) were added to per Eppendorf tube, cells were vortexed, incubated for 15 min at room temperature in dark. Cells were analyzed by flow cytometry on an ACEA Novo cyte 1000 (ACEA Biosciences Inc., San Diego, CA, USA), PI binds to DNA when the cell membrane is disrupted and AV binds to translocated phosphatidylserine on the outer surface of the cell membrane (early apoptosis signal). Percentage of early apoptotic and late apoptotic/necrotic cell populations were calculated using Nove Express software (version 1.2.1, ACEA Biosciences Inc., San Diego, CA, USA) and compared with appropriate controls.
Differentiation Marker Analysis
The expression of CD235a (an erythrocyte differentiation marker) antigen on the surface of HEL cells were measured by flow cytometry. Cells were plated in 60 mm dish in 3 mL medium at 1×105 cells and exposed to different concentration of GZWM- 060 (0.15 to 1µmol/L) for 24h. After treatment, cells were washed with PBS twice, then incubated with CD235a (CD235a-APC) for one h in the dark at 4°C. The cells were washed twice with PBS and finally resuspended in 200 µL PBS for measurement. CD235a expression levels were measured using flow cytometry (ACEA Biosciences Inc., San Diego, CA, USA).
Cell Cycle Measurement
HEL cells were seeded in 6-well culture plates at a density of 1×10 5 cells in 2 mL medium and were treated with GZWM-060 for different concentration (0.15 to 1 µmol/L) for 18 and 24h. After the incubation, the cells were collected and transferred into a sterile centrifuge tube for cell cycle analysis. Cells were washed with precool PBS and suspended in 70% ice-ethanol, incubated 3~4 h at 4°C and preserved in a refrigerator at -20°C overnight. To remove the stationary liquid, the cells were centrifuged and washed twice with cold PBS. Then, 500 µL mix dye solution (RNas eA 100µg/mL, PI 50 µg/mL, Triton X -100 0.2%) were added into each tube, gently mixed and incubated for 10 min at room temperature in the dark. Before analysis by using flow cytometry, the cells were washed with cold PBS, and 200 µl suspension were used analysis by a Novo Cyte flow cytometer (ACEA Biosciences, Inc., San Diego, CA, USA) using Novo Express 1.0.2 software. Each experiment was conducted three times.
GZWM-060 treated GZWM-060cells in different concentration (0.15 to 1µmol/ L) for 16 h, cells were collected. Total RNA was extracted by Trizol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) and quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, USA). Complementary DNA was synthesized in a 20μl reaction containing one μg of total RNA, using PrimeScript™ RT reagent Kit (Takara Bio Group) according to the manufacturer’s instructions. To examine the quality, the cDNA was amplified by the beta-actin gene using the Taq Master Mix (Tiangen Beijing, China), and 1% agarose gel electrophoresis was performed. Subsequently, to determine the mRNA levels of miR17, miR18a, miR19a, miR19b, miR 20a, miR 92, qRT-PCR was performed by using SYBR Green qPCR Master, the quantitative analysis of the change in expression levels was calculated by ABI 7300 real-time PCR machine (Applied Biosystems, Carlsbad, CA), the cycling condition was as follows, 95 °C for 15 min followed by 40 cycles of 95 °C for 30s and 60°C (primer TM) for 30s, 72°C for 60s. All data were controlled for the quantity of RNA input by performing measurements on the endogenous reference gene β-actin. As well as, RNA results from treated samples were normalized to results obtained using RNA from the control. An average of three experiments each performed three times with standard errors is presented. Primer quality was analyzed by dissociation curves. Primer sequences are listed in Table 1. Data were analyzed by comparing Ct values.
Western Blot Analysis
Cells were treated by GZWM-060 in different concentration (0.15 to 1 µmol/L) for 18 h, cells were extracted and total protein was collected from cells in RIPA lysis buffer. Protein’s concentration was determined by the level of protein BCA test kit (Solarbio life sciences, China), and proteins were separated by 10% SDSPAGE, then blotted onto PVDF membrane (0.22 µm, Merck KGaA, Germany). The membranes were incubated in solution with 3% BSA (dissolved in TBST) at room temperature for 1h and probed with primary antibodies STAT3(1:1000), p-STAT3(1:1000), Bim (1:1000), and GAPDH (1:2000) at 4°C overnight. After washing with TBST 3 times, the membrane was incubated with secondary antibody. Finally, immunoreactive protein signals were detected using the Odyssey Infrared Imaging System. GAPDH was used as an internal loading control. Shown are representative data from individual experiments that were repeated at least twice.
Statistical Analysis
Experimental data were valued by a mean ± standard deviation of three independent assays. All tests were carried out three times. An independent test was conducted for comparison between groups. Statistical significance was determined using Student’s t-tests. Statistically different values were defined significant at *P < .05, **P < .01, and ***P < .001.
Results
Cytotoxic Effects of The Compound GZWM-060 On HEL Cells
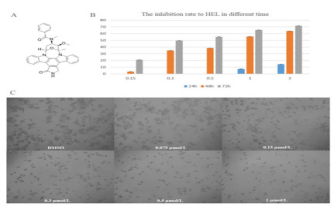
Midostaurin (PKC412), a semi-synthetic derivative of staurosporine has a strong ability to kill leukemia cells in patients with positive oncogenic FLT3. To obtain more effective candidates’ drugs for the treatment of leukemia patients. We synthesized many compounds from the skeleton of PKC412 and evaluated the cytotoxicity of these compounds on the human leukemia cell line (HEL) by using MTT assay. The observed results demonstrated that the GZWM-060 showed strong anti-cancer activity against HEL. (Table 2) The observed IC50 value of GZWM-060 against HEL was 0.3558±0.0673 µM (72h), and the IC50 value of the positive drug PKC412 against HEL was 0.6638±0.3667 µM (72h). To observe whether GZWM-060 could inhibit the growth of HEL cells in a dose- and time-dependent manner, the HEL cells were treated with different concentration of GZWM-060 (0.15, 0.3, 0.5, 1, 3 µmol/L) and incubated for different time periods at 24, 48, 72h in three independent assays to detect the cell viability. The results showed that the compound GZWM-060 could inhibit HEL growth in a doseand time-dependent manner. Interestingly, the compound exerted higher effective against HEL cells growth than PKC412 (Figure 1). response curve of the HEL cell line derived from MTT cytotoxicity assay performed; C. Morphological changes of the leukemia cell line of HEL treatment with different concentration of GZWM-060 in 72h. (Table 3) Data are presented as mean ± standard error (n=3).
Figure 1: The inhibition rate to HEL cell.
A. The chemical structure of PKC412;
B. The dose-time– response curve of the HEL cell line derived from MTT cytotoxicity assay performed;
C. Morphological changes of the leukemia cell line of HEL treatment with different concentration of GZWM-060 in 72h. Data are presented as mean ± standard error (n=3).
The Compound GZWM-060 Induced HEL Cells Apoptosis
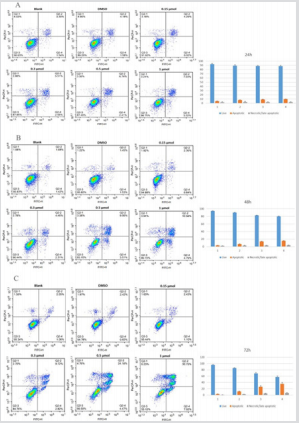
Apoptosis is central to block cancer cells growth. To study whether the compound GZWM-060 block HEL cells growth through inducing apoptosis, the effects of GZWM-060 on cell apoptosis were investigated by flow cytometry after staining with Annexin V and propidium iodide. The results showed that the treatment with GZWM- 060 for 24 h, 48 h, 72 h significantly induced apoptosis in HEL cells in a dose- and time-dependent manner (Figure 2).
Figure 2: The apoptosis result of HEL cell in different time at different concentration of GZWM- 060 (0.15–1µmol/L).
A. The apoptosis result of HEL cell in 24h
B. The apoptosis result of HEL cell in 48h
C. The apoptosis result of HEL cell in 72h
The Compound GZWM-060 Induced HEL Cells Differentiation into Erythrocytes the Induction of Terminal Differentiation in Cancer Cells Is A Central Way to Arrest
cancer cells growth. The human erythroleukemia cell line (HEL) is an established model to study erythroid and megakaryocytic differentiation in response to small molecular stimulation in vitro [13]. To determine whether the compound GZWM-060 block cells growth through inducing differentiation in HEL cells. As a result, the flow cytometric analysis demonstrated that the expression of CD235a (an erythrocyte differentiation marker) of the HEL cell was increased after treatment with GZWM-060. Therefore, the data exposed that GZWM-060 might have a substantial effect on inducing HEL cells differentiation, and the up-regulation of differentiation marker present a dose-independent manner (Figure 3A).
The Compound GZWM-060 Arrested HEL Cell Cycle
To identify whether the growth inhibitory effect of HEL cells caused by specific disruption of the cell cycle-related event, the cell cycle phase distributions was measured using a flow cytometric analysis. HEL cells were treated with GZWM-060 at different concentration for 18 h and 24 h. The observations revealed the number of cells in the G1 phase significantly decreased in both cell lines, and the G2 phase significantly increased, which exerted a dose-independent manner (Figure 3B).
Figure 3: The results of flow cytometry.
A. The differentiation results of CD235a in HEL cell at different concentration of GZWM-060 (0.15–1µmol/L.
B. The cell cycle result of HEL cell in different time (18h, 24h) at different concentration of GZWM-060 (0.15–1µmol/L).
The Compound GZWM-060 Inhibited the Expression of Mir-17-92
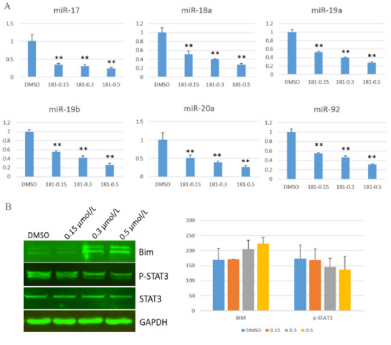
MiR-17-92 plays a vital role in activating cancer cells proliferation [14]. To evaluate whether the compound GZWM-060 inhibit the expression of miR-17-92 in HEL cells, the expression of miR-17-92 was analyzed in HEL cells treated with the compound GZWM-060 by using qRT-PCR. miR17, miR18a, miR19a, miR19b, miR20a, miR92 were significantly down-regulated after treated with GZWM-060 compared with DMSO control group (Figure 4A).
The Effects of The Compound GZWM-060 on Proteins of Down or Up-Stream of Mir-17-92
Our previous work indicated that STAT3 could enhance the expression of miR-17- 92 by binding its promoter, and BIM is a target of miR-17-92 [15]. BIM and p-STAT3 are the critical regulators of apoptosis and cell cycle proteins. To validate the effects of GZWM060 on p-STAT3 and BIM protein levels in HEL cells, we examined protein levels of BIM and p-STAT3 by using western blot. The results showed that the protein level of BIM was up-regulated, but the p-STAT3 level was down-regulated in HEL cells treated with the compound GZWM-060 (Figure 4B).
Figure 4: The result of Qrt-PCR and western blot.
A. The expression level of miR17-92 IN HEL cell with different concentration of GZWM-060 (0.15-0.5μmol/L;
B. The expression level of protein with different concentration of GZWM-060 (0.15-0.5μmol/L).
Discussion
To meet the continuous demand, a constant and sufficient supply of anticancer drugs is essential. Although many compounds possess anticancer effects, the underlying molecular mechanisms remain to be established. PKC 412 was first synthesized in 1986 [16] and was initially developed as a small molecular inhibitor of protein kinase C (PKC) [17]. Studies to investigate its potential as a PKC inhibitor revealed that it inhibited cell proliferation by interfering with cell-cycle activity [18,19]. Understanding the antitumor activity of mechanism can facilitate the foundation of a new target for treatment. In our study, we synthesized the compound GZWM-060 from the skeleton of PKC412 and found that GZWM060 could inhibit cells proliferation and exerts cytotoxicity against human leukemia HEL with an IC50 value of 0.3558±0.0673 µM. Further studies indicated that GZWM-060 induced apoptosis and arrested the cell cycle in leukemia cell, promoted the differentiation into erythrocytes. These results showed that GZWM-060 might be a lead molecule for the development of new anti-cancer drugs candidates.
Apoptosis is a highly synchronized and conserved cellular phenomenon maintained by a highly organized network of intrinsic cellular suicide machinery. When the homeostasis between cell proliferation and death is disturbed, apoptosis-inducing pathways are altered, which results in oncogenesis [20]. The induction of apoptosis in cancer had been identified as a target for the treatment of cancer [21, 22]. There have two distinct signaling pathways of apoptosis, the intrinsic or mitochondrial pathway, and the extrinsic or death receptor pathway [23, 24]. The regulation of these apoptotic mitochondrial events occurs through members of the Bcl-2 family of the protein [25] and is classified into one anti-apoptotic and two pro-apoptotic groups, which BIM belong to the pro-apoptotic proteins [23]. The western blot results show that the molecular mechanism of apoptosis induction by GZWM-060 includes modulation of the protein expression of BIM. BIM-induced apoptosis is critical for the development and homeostasis of immune cells [26]. In the past decade, BIM has been recognized as an essential pro-apoptotic protein for initiating the intrinsic apoptotic pathway under many physiological and pathophysiological conditions [27], De Bruyne discovered that BIM promoter acetylation was linked to increased BIM expression in multiple myeloma and aggravated apoptosis [28].
The western blot experiments revealed that p-STAT3 was inhibited by GZWM-060, with exposure to GZWM-060 at 0.15, 0.3 and 0.5µM, the level of phosphorylated STAT3 (p -STAT3) was decreased compared to the untreated group. The reduced expression of p-STAT3, as well as increased cell apoptosis, presented themselves in a time and concentration-dependent manner. Progression by the cell cycle phases (G1, S, G2, M) is under the control of a family of serine/threonine protein kinases [29]. BIM plays a vital role in the regulation of cell cycle. Activating BIM lead to cells growth inhibition at specific stages in the cell cycle [15]. The anti-proliferative activity of GZWM-060 also involves an increase in BIM protein level, which disruption of cell cycle process plays a role in the anti-oncogenic. BIM also binds to and represses the transcription factor signal transducer and activator of transcription 3 (STAT3), thereby inhibiting cytokine-stimulated and STAT3- dependent gene expression [30], that consistent with our results. The family of STAT protein includes seven transcription factors: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6 [31].
STAT3 has been noticed as one of the critical factors for tumors formation [32] and has a crucial role in breast cancer initiation [33]. In natural cells, STAT3 activation is tightly controlled, but activated STAT3 (p-STAT3) has been identified in many kinds of human tumor samples and cancer cell lines, which indicates it plays a pivotal role in the process of tumor development [34]. Recent studies have demonstrated that p-STAT3 overexpression is associated with poorer prognosis in patients with some cancer [35-37], and some studies indicated that p-STAT3 enters into the nucleus and promotes cell proliferation, drug resistance and suppresses tumor cell apoptosis [38,39]. MicroRNAs are types of small non-coding RNAs whose mature products are ~22
nucleotides long, which get involved in various biological processes including the cell cycle, differentiation, growth, and development, metabolism, aging and apoptosis [40,41]. In 2005, He et al. first discovered the miR-17-92 cluster, an oncogenic gene in human B-cell lymphomas [42]. The miR-17-92 cluster is a typical highly conserved polycistronic miRNA cluster, which is located in the human chromosome 13, encoding six mature miRNAs, including miR-17, miR-18a, miR-19a, miR-19b, miR-20a and miR-92a [43].
MiR-17-92 play an essential role in both the apoptotic and cellproliferation pathways [44,45]. Jack’s lab showed that deletion of miR-17-92 could induce up-regulation of the pro-apoptotic protein BIM by using miR-17-92 knockout mice [46]. Li et al. discovered that miR-17-92 could activate BIM when they knocked down miR92, cell cycling was arrested at G1/S via up-regulation of BIM [15]. Besides, it has been demonstrated that the miR-17-92 cluster can inhibit the expression of the tumor suppressor and the apoptotic gene BIM in lymphoma [47]. In our study, the result of qRT-PCR indicated that the expression of miR17-92 down-regulation by GZWM- 060 and western blot showed that both of the expression of BIM was increased, so GZWM-060 maybe as a drug candidate by targeting to the STAT3/miR-17-92/BIM signal pathway
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81700169, 81502959, 21502034, 30572246 and 30973680), the Natural Science Foundation of Guizhou (QKH 20181409), the 100 Leading Talents of Guizhou Province (fund for Y. Li and W. Zhu), QKHPTRC2017 (5737), Light of the Western Talent Cultivation Program of the Chinese Academy of Sciences (201684).
Disclosure Statement
The authors declared no conflicts of interest. W. Zhu designed and L. Wang synthesized the compound, GZWM-060.
For more Article: https://biomedres01.blogspot.com/






No comments:
Post a Comment
Note: Only a member of this blog may post a comment.