Quantification of Flavonoides and Phenols of Bravo Bean Extract (Capparis flexuosa L. Caparaceae)
Introduction
The use of plants for medicinal purposes is not present only
in contemporaneity, since the course of human history there was
already the search for plants that possessed the curative capacity,
even with little knowledge about them, the men of antiquity
already used them and distinguished them for the various
purposes. Medicinal herbs are fundamental in the treatment of
various pathologies, especially in communities isolated from large
urban centers [1]. With the advent of navigations it was possible
to discover new continents, presenting to the world the great
therapeutic arsenal derived from plants, indispensable to modern
medicine [2]. Nature has always been a source of charm for man,
not only for the useful resources of its food and maintenance, but
for being its greatest source of learning and inspiration [3]. Brazil is
a privileged country in biodiversity since, of all the plant species in
the world, 20% is in The Brazilian territory. Several native or nonnative
species face risk of extinction due to the degradation of their
habitat and exploratory [4].
Although Brazil has a great biodiversity, there are still few species cataloged and strictly studied as their therapeutic potential, which makes it difficult to make use of these. However, ethnobotany research in the Northeast has shown satisfactory results, especially of plants adapted in the caatinga [5]. The caatinga biome is present only in The Brazilian territory, with an area of 844,453km2, which corresponds to 11.00% of the national territory, predominant of all states of the Northeast and North region of Minas Gerais [6]. This biome has long been considered poor in biodiversity, however, studies have shown its rich biological diversity. More than 2,000 species of vascular, succulent, woody plants and various species of bees, amphibians, birds and mammals have been recorded. Among several species of caatinga, several plants have antioxidant action and several other biological activities, and are used as a remedy for popular use, with leaves, stems and seeds sold in markets and free fairs [6,7-9].
Thus, it is possible to emphasize the species belonging to the family Caparaceae more precisely the species Capparis flexuosa L. (Caparaceae), which is popularly known as wild beans, stands out for presenting a favorable development in semiarid regions, such as the northeast, and in a dry period the occurrence by biological production is satisfactory of this species. Its morphological characteristics consist of a shrub-tree size of perennial leaves, in the late afternoon insects and bees become attracted by their flowers, because they have the ability to ezalarem odor, more precisely due to the main floral resource that it has nectar [10]. Due to its meliferous relevance, C. flexuosa L. is recommended to be cultivated in regions that have as activity the creation and conservation of bees, for the extraction of honey. In addition, this forage exercise an important activity in cattle ranching activities in the northeast, which serve as food for the herds, mainly for sheep and goats, thanks to their resistance to develop in dry periods [11].
In traditional medicine, wild beans are presented as a contributor to several diseases, such as: sinusitis through its inhalation, and against snake bite reactions, from the embehest of the liquid resulting from its peel shaved with water. In view of the attributions of wild beans in traditional medicine, it is important to quantify phenolic compounds and flavonoids of the leaves of C. flexuosa, given the great biological and pharmacological importance of these bioactive compounds in the human organism [12-15]. Therefore, the objective of this study was to quantify the total phenol and flavonoid content of the leaves of C. flexuosa L, using spectrophotometric techniques.
Methodology
Obtaining the Crude Ethanol Extract
The leaves were collected, identified, dried at room temperature and subsequently crushed. The extraction of the chemical constituents was performed by macerated in P.A. ethanol for two weeks. The extractor liquid was concentrated in rotaevaporator at a temperature of 45 °C degrees Celsius, to obtain the crude extract.
Phenolic Compounds - Folin Ciocalteau
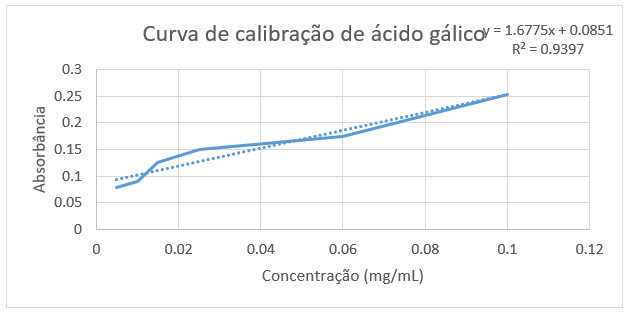
The quantification of phenolic compounds and flavonoids was performed by the analytical method of quantification by external standardization, with the aid of a UV-VIS spectrophotometer. The method for determination of total phenols was adapted from [16], and consists of the reaction of the constituent acids of the folinciocalteau reagent and phenolic or non-phenolic compounds. To quantify the phenol content, a calibration curve of manic acid was performed with concentrations ranging from 0.15 to 0.005 mg/mL. After preparing the calibration curve, the plant extract solution was prepared. Initially, 0.005 g of the plant sample was weighed and diluted in 5 mL of MeOH. Then, a rate of 0.075 mL of this solution was removed and 0.425 mL of MeOH (stock solution) was added. To perform the reading, 100μL of the stock solution, 500μL of folin - Ciocalteau and 6 mL of Distilled H2 for each sample were added in amber glass (in triplicate- for eachsample). It was subsequently stirred in the vortex for 1 minute, then 2 mL of 15% sodium carbonate was added and stirred again in the vortex for 30 seconds. The last procedure for the preparation of the solutionswas transferred to the solution to a 10 mL volumetric balloon and the volume was completed with Distilled H2O; and then the solutions were incubated in the dark for 2 hours. To obtain the white, a solution of 100μL of MeOH, 500μL of the Reagent Folin-Ciocalteau and 1 mL of H2The distilledwas prepared. Soon after, 2 mL of sodium carbonate was added 15% and then stirred for 30 seconds in the vortex. Subsequently completed the volume of the solution in a 10 mL volumetric balloon. The solution was incubated in the dark for 2 hours. Before any reading, the whitepp p zeroe the spectrophotometer was used. A UV-VIS spectrophotometer with a wavelength of 750 nm was used to read the solutions.
Quantification of Flavonoid Content
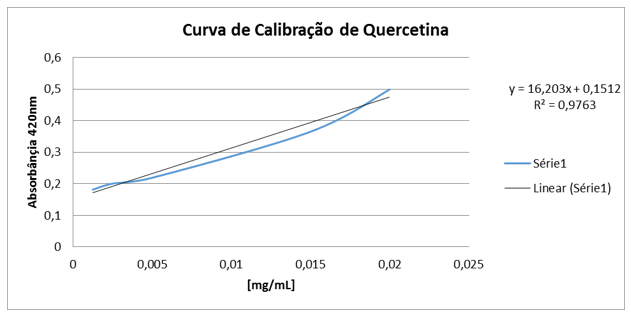
The analysis was adapted to the study by [17]. Initially, the calibration curve was prepared with the quercetin pattern, where 1mg of quercetin was weighed and diluted in 1ml of MeOH. Then, dilutions were performed at concentrations of 0.03; 0,025; 0,020; 0,015; 0,01; 0,005; 0.0025 and 0.00125mg/ml. Soon after, it weighed it and 1mg of the crude extract and diluted it in 1ml of MeOH. After preparing the stock solution, the solutions (in wells - in triplicate) were made for reading that contained 200μl of the test solution of the plant sample and 100μl of 2% aluminumchloride solution. The solution for white (in triplicate) was prepared with 200μl of MeOH and 100μl of 2% aluminum chloride methanic solution. Then the well plate was kept in the dark for 30 minutes. After the time, the reading was performed in aPECTrophobimeter UV-VIS at 420nm. The flavonoid content was determined by interpolation of the average absorbance of the samples against the quercetin calibration curve (replacement of the straight equation) and expressed in mg of EQ (quercetin equivalent) per g of the extract.
Results and Discussion
Phenolic substances are widely distributed in nature, about 8000 phenolic compounds have been found in plants [18]. These compounds can be classified according to their chemical structure, in classes such as simple phenols, phenolic acids, acetophenones, phenylaacetic acids, hydroxycinamic acids, phenylpropenes, coumarins, xanthones, anthraquinonas, flavonoids among others [19]. One of the most importantclasses of phenolic compounds are flavonoids. This class is widely distributed in the plant kingdom, being found in fruits, seeds, roots, splints, flowers, teas and wines. They are aromatic substances and have basic structure C6-C3-C6, with 15 carbon atoms. The two rings are aromatic (rings A and B) joined by three carbons that form a heterocyclic with ring C [20]. By the Folin–Ciocalteau method, the total phenol content of the extract of C. flexuosa leaves was determined by the spectrophotometric method. The total phenol content was identified by interpolation of the absorbance of the samples against a calibration curve constructed with patterns of lalic acid [21], as shown in Graph 1.
By interpolating the absorbances of the species C. flexuosa, a content of 1916.9 mg EAG/g of extract was obtained; a result being superior to others already described in the literature. According to JUNIOR (2015) [15], when analyzing the wild bean, a phenol content of 595.47 mg EAG/g of extract was obtained. Assim as phenolic compounds, flavonoids also play an important role in the protection of living organisms against oxidative agents, present in plants, which act as bioactive agents, such as anti-inflammatory, antiallergic, anti-hemorrhagic, action against diabetic retinopathy and among others, highlighting its performance mainly as antioxidant [8,13,14]. Thus, there was a quantitative analysis of the flavonoid content of the species C. flexuosa, obtaining a content of 84.04 mg EQ/g of extract, byinterpolating the absorbances of the sample with a standard quercetin curve (Graph 2).

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.