Predictors of Mortality Among Children Co-Infected with Tuberculosis and Human Immunodeficiency Virus in Region, North Ethiopia, Retrospective Follow- Up Study
Background
Tuberculosis (TB) and human immunodeficiency virus (HIV)
co-infection remain a major global and national health problem
that requires substantial action to achieve the Sustainable
Development Goals (SDG) and the END-TB strategies [1]. Both TB
and HIV are the leading causes of death from infectious diseases
worldwide [2]. Mycobacterium tuberculosis and HIV co-infection in
the human body, potentiate each other and accelerate to death by
deteriorating body immunity causing premature death if untreated
[3]. Tuberculosis is a major cause of morbidity and mortality in
HIV-infected children [4]. In 2015, the World Health Organization
(WHO) report showed that nearly 41,000 children died from TB and
HIV co-infection. Of which more than 83% were occurred in Africa
[5]. Mortality among children co-infected with TB and HIV varied in
different settings and fluctuated widely from 6.2% to 36.5% [5-7].
In Ethiopia, mortality of children co-infected with TB and HIV was
14% [8] and co-infected children had six times greater death than
TB disease alone [9]. Furthermore, more than 1 in 5 TB and HIV coinfected
individuals were died [10], but this huge problem was not
specifically known in children.
The prevalence of TB and HIV co-infection in children was
under-assured due to the problem of reaching a definitive
diagnosis. However, the WHO report showed that HIV prevalence
among children with active TB disease ranges from 10 to 60%,
depending on the background rates of HIV infection in countries
with moderate to high prevalence of TB [11]. The estimated rates of
tuberculosis among HIV positive children also had a wide variation,
depending on the TB epidemic and the coverage of highly active
antiretroviral treatment (HAART) coverage in the area [4].
Data on the survival of TB and HIV co-infection in children
are still lacking and the available information is difficult to
interpret due to problems with the diagnosis and selection of
study populations [4]. In developing countries, including Ethiopia,
the management of TB and HIV co-infection in children is very
challenging due to the inaccessibility of appropriate formulations
of drugs, drug-drug interactions, pill burdens, drug side effects, and
poor drug adherence [12-14]. This may result in high TB incidence
and mortality among HIV-positive children. TB is not only the most
commonly reported opportunistic infection [15], but also a major
cause of hospital admission and death in HIV infected children
[16]. The cause of death is also multifactorial and determined by
socio demographic, clinical, laboratory, drug and follow-up related
factors [8]. Which are poorly understood. Therefore, studies on
mortality and its predictors in TB and HIV co-infection in children
are very significant to designate appropriate action according to
their ages.
Most of the studies on TB-HIV co-infection focused on adult,
fewer studies on general co-infected population, little is known in
pediatrics sub-age group. Still, the problem in children is masked
and actions are taken based on findings from studies in the adult
population. However, the problem is very alarming in children
due to immature immune system and fast deterioration into death
[17,18]. A previous study in the comprehensive specialized hospital
of Gondar University in Ethiopia lacks a time specification on the TB
and HIV co-infection period, rather they prolonged their follow-up
after TB was cured. This makes the study more biased.
To some extent, there is better evidence on the incidence and
predictors of tuberculosis in HIV-infected children [19,20], but
evidence on survival and mortality after co-infection is limited in
Ethiopia. Therefore, survival and predictors of mortality among
children co-infected with TB and HIV have not been well documented
in Ethiopia. Therefore, this study was to try to fill the above gaps by
estimating survival and identifying predictors of mortality among
children co-infected with TB / HIV in public general hospitals in
Mekelle and the southern zone of Tigray region, northern Ethiopia.
Methods
Study Design, Setting, and Period
A retrospective hospital follow-up study was conducted in two zones of the Tigray Region (Mekelle and Southern), which is located in the northern part of Ethiopia by reviewing 10 years (2008- 2018) medical records of children co-infected with TB and HIV in 2019. About 1,179,687 populations lived in these two zones. Of which 515,524 were children [21]. The study was conducted from October 1,2018 to June 30, 2019 in three selected general hospitals (Mekelle, Alamata, and Maychew).
Population and Sampling
Source Population
All children infected with TB and HIV co-infected under 15 years of age who received follow-up care from January 1 / 2008 to December 30/2018 in the ant-retroviral treatment (ART) clinic at public general hospitals of the Mekelle and southern zone of the Tigray region, North Ethiopia.
Study Population
All children co-infected with TB and HIV, under 15 years of age and those who followed up from January 1 / 2008 to December 30/2018 in the ART care clinic of selected hospitals in the study area.
Inclusion and Exclusion Criteria
Children infected with TB-HIV co-infected younger than 15 years were included in this study and had follow-up care from January 1/2008 – December 30/2018 in a selected hospital. Children who had missed key information on clinical, immunological, drug information and their outcomes had not been recorded on medical charts were excluded.
Sampling Technique
In the Mekelle and Sothern zones of the Tigray region, five general hospitals were found to provide ART services. These are the general hospitals of Mekelle, Quiha, Maychew, Alamata, and Korem. However, this study used cluster sampling by randomly selecting three hospitals (Mekelle, Alamata, and Maychew). Since we used cluster sampling, all children co-infected with TB and HIV who were enrolled in selected hospitals in two zones who met the inclusion criteria were included. The medical charts of children with TB and HIV co-infected from 2008 -2018 were reviewed.
Data Collection and Analysis
Data were collected from medical records (charts) using a data
extraction checklist developed from the national HIV intake and
follow-up form [22]. The checklist consisted of sociodemographic,
clinical, and HIV care/ART/ follow-up related information. Data
were collected from April 15/2019 to May 20/2019 from medical
records. If the child is co-infected with TB and HIV, the follow-up
should continue for the entire life (for HIV care) even if the child was
cured from TB. After verifying completeness and consistency, the
data were coded and entered into Epi-data manager version 4.4.2.1
and then exported to Stata version 14 for analysis. Kaplan–Meier
survival graph and Log-rank test were used to compare the survival
difference between intragroups of categorical variables. Mortality
rate, person-time observation, and mean survival time were
calculated by Stata. The Cox proportional hazard model was used
for analysis. The Schoenfeld residual test (estat phtest) or global
test was used to check the Cox proportional hazard assumption, it
was non-significant (Prob>chi2 = 0.4179) indicates the hazard was
proportional over time. Regarding multi- collinearity, the mean VIF
was 1.39 indicates, collinearity between variables was within the
acceptable range.
Both bivariate and multivariate analysis was computed to
determine the association between predictor variables and the
outcome variable. These variables that were significantly associated
with a p-value of <0.2 in the bivariate analysis were entered into
the multivariate analysis. Variables significantly associated with
the outcome variable at a p-value <0.05 in the multivariate analysis
were considered independent predictors of mortality. Finally, the
adjusted hazard ratio with 95% CI and P value was used to measure
the significant association between predictors and outcome
variable.
Ethical Considerations
The study protocol was evaluated and approved by the Institutional Review Board (IRB) of Mekelle University, a college of health sciences, and then ethical clearance was obtained. A cooperation letter was written to the chief executive managers of each hospital. Since the study was retrospective and document review, it did not cause any risk to the study participants.
Results
Sociodemographic Characteristics
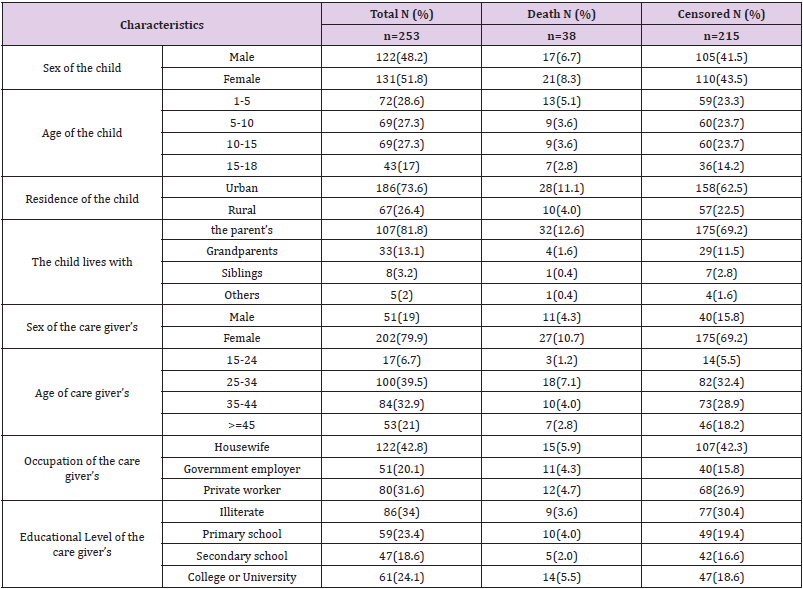
A total of 282 children with co-infected TB and HIV were enrolled in the general hospitals of Mekelle, Alamata, and Maychew. Of which 29 were excluded from the study due to lost cards or incomplete data. The remaining 253 children co-infected with TB and HIV were included in the study. The median age of the study participants was 8 years with IQR (4-13). One hundred and thirtyone (51.8%) of the children were females (Table 1).
Table 1: Sociodemographic characteristics of children co-infected with TB and HIV in general hospitals of two zones of the Tigray region, North Ethiopia, 2019 (n=253).
Clinical and Immunological Related Characteristics
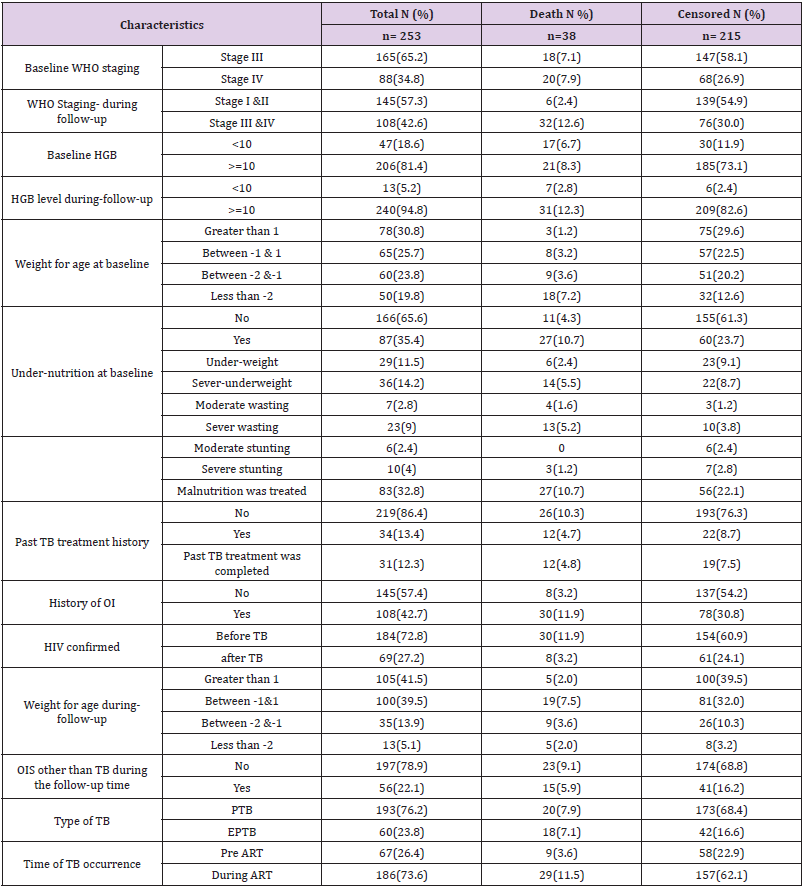
Of a total of 253 children co-infected with TB and HIV, 186 (73.6%) of them developed TB after starting ART. At baseline, 165 (65.2%) of the children co-infected with TB and HIV had WHO stage III, and 129 (51%) had a CD4 count of less than 350 with a median of 330 cells (IQR (176.50-519.50)) cells/μl. During followup, 145 (57.3%) of the children co-infected with TB and HIV had improved their WHO staging to stage I & II. However, 66 (26.2%) of the children had a CD4 count of less than 350 with a median of 540 IQR cells (322.50-840.50) cells/μl. Thirteen (5.2%) of the children had anemia (HGB <10mg/dl) with a median HGB level of 13 (IQR (12-14.4)) mg/dl (Table 2).
Table 2: Clinical and immunological characteristics among children co-infected with TB and HIV in general hospitals of two zones of the Tigray region, North Ethiopia, 2019 (n=253).
Education and Follow-Up Related Characteristics
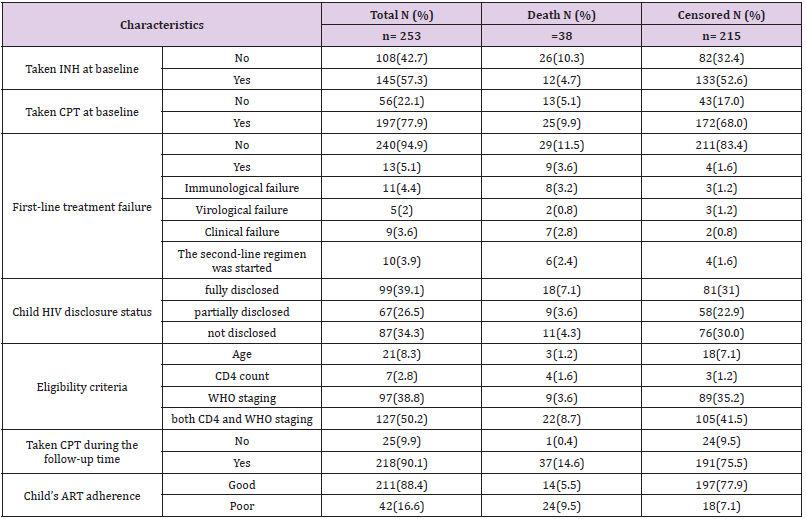
One hundred and ninety-seven (77.9%) of the respondents had taken co-trimoxazole preventive therapy and 145 (57.3%) had also taken isoniazid preventive therapy before developing TB. The initial ART regimen was changed in 59 (23.3%) of the children due to side effects 35 (13.9%), TB 9 (3.6%), treatment failure 13 (5.1%) and other reasons 4 (1.6%) such as drug toxicity. Firstline ART treatment failure was observed in 13 (5.1%) children. Of these, 10 (76.9%) of them initiated second-line ART regimens. Regarding ART adherence, 211 (88.4%) of the children had good ART adherence (Table 3).
Table 3: Medication and follow-up related characteristics among children co-infected with TB and HIV in general hospitals of two zones of the Tigray region, North Ethiopia, 2019 (n=253).
The Mortality Rate Among Children Co-Infected with TB and HIV
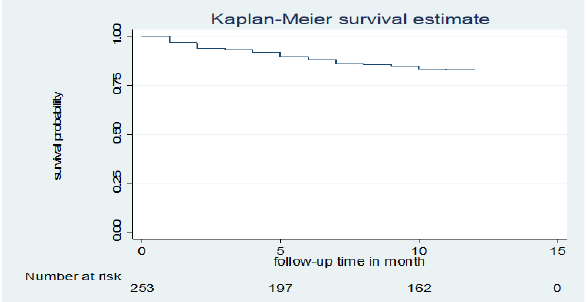
Of a total of 253 children co-infected with TB and HIV included in the study, 38 (15%) deaths and 215 (85%) censored were recorded. Of the censored cases, 186 (73.5%) were alive until the end of the follow-up period, 14 (5.5%) were transferred out, 15 (5.9%) were dropped out of follow-up, and the rest were in TB treatment. Those 253 TB and HIV co-infected children were followed for different periods (1 month to 12 months), which provides 226 child-month observations with a mean survival time of 10.75 (95% CI; 10.37 -11.14) months. In this study, the mortality rate was 0.17 (95% CI 0.12 to 0.23) per 1,000 child-month observations. The majority (73.7%) of the deaths occurred in the first six months of followup period and 15 (40%) occurred during the initial phase of TB treatment. All deaths 38 (15.02%) had occurred during ART. The cumulative probability of survival at the end of 2 months, 6 months, 9 months and 12 months was 94.0 %, 88.0%, 85.0 % and 82.9%, respectively (Figure 1).
Figure 1: Kaplan-Meier cumulative survival estimate of children co-infected with TB and HIV in general hospitals of two zones of the Tigray region, North Ethiopia, 2019.
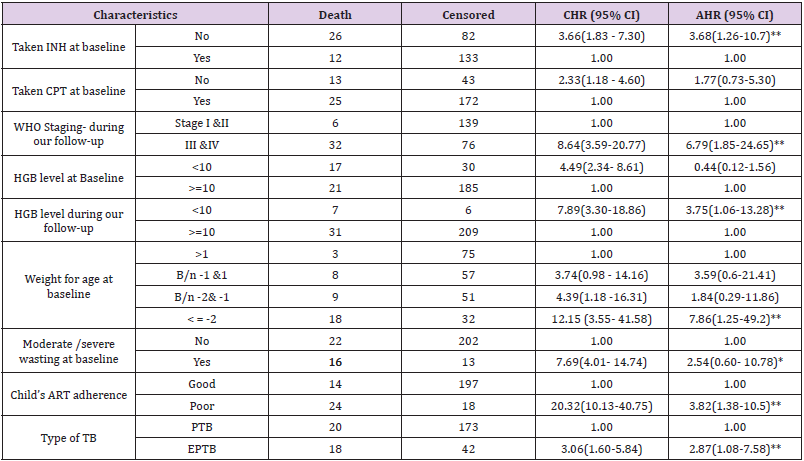
Predictors of Mortality Among Children Co-Infected with TB and HIV
Bivariate and multivariate analyzes were used to assess
the significant association between exposure variables and the
outcome variable. Underweight at baseline, moderate / severe
wasting at baseline, IPT, CPT, baseline hemoglobin level, level
of adherence to ART, type of tuberculosis, WHO staging during
follow-up, and hemoglobin level during follow-up were statistically
significant at 0.2 level of significance in bivariate analysis. In
multivariate analysis; underweight at baseline, IPT user/not/, ART
adherence level, type of TB, WHO staging during follow-up, and
hemoglobin level during follow-up were statistically significant at
0.05 significance level (Table 4).
The risk of death among children with TB and HIV co-infected
with underweight was approximately 8 times higher than children
with normal weight at baseline (AHR=7.9 (95% CI 1.26, 49.3)).
Children who did not take IPT were approximately 4 times more
likely to experience death than children who had taken IPT (AHR=3.69 (95% CI=1.26, 10.8)). The risk of child death with poor
adherence to ART was approximately 4 times higher than children
with good adherence to ART (AHR = 3.82 (95% CI: 1.38, 10.54)).
The risk of death among children infected with extrapulmonary
TB was also approximately 3 times higher than infected children
with pulmonary TB (AHR = 2.9 (95% CI: 1.1, 7.6)). During
follow-up, children with advanced WHO staging (III & IV) were
approximately 7 times higher risk of death than children with stage
I and II (AHR=6.79 (95% CI= 1.85, 24.9)). Anemic children were
approximately four times more likely to experience death compared
to nonanemic children during follow-up (AHR=3.76 (95% CI= 1.06,
13.27)).
Table 4: Results of the bivariate and multivariate analysis among children infected with TB and HIV in general hospitals of two zones of the Tigray region, North Ethiopia, 2019(n=253).
Discussion
The study provides information on the overwhelming problem
of high mortality and associated predictors among children with
TB and HIV coinfected. The mortality rate in this study was 0.17
(95% CI 0.12–0.23) per 1000 child-month observations. The result
was lower than the mortality rate reported from a single study
conducted in four developing countries (Burkina Faso, Cambodia,
Cameroon and Vietnam), which is 0.370 per 1000 child- month
observations [23]. The difference may depend on the sample size
difference used by the studies.
In this study, mortality was higher in underweight children at
baseline. A similar finding was reported from a study conducted
in Thailand [24]. This might be the effect of underweight on
reducing body metabolic processes resulting in inadequate energy
acquisition that increases disease progression, which may end up
in death. Furthermore, inadequate weight gain in TB treatment
indicates a poor response to treatment [25]. However, stunting and
wasting were not significant in this study. This could be due to a
higher proportion (90%) of children diagnosed with malnutrition
in this study who received treatment for malnutrition. The study
also revealed that children who did not take IPT were three times
more likely to experience death than children who did take IPT.
This was in line with a study conducted in Gondar, Ethiopia [8].
The possible reason might be that IPT reduces the severity and
spread of TB disease. However, CPT was not found to be statistically
significant in this study, which was reported as a protective factor
for death in a study conducted in Gondar, Ethiopia [8]. This may
be because a higher proportion (78%) of our respondents had
taken CPT and were unable to make a difference. The number of
children who didn’t take CPT and died was too few (5.1%). For
better survival, HIV positive children should take both CPT and IPT
as preventive prophylaxis. In this study, the risk of death among
children infected with extrapulmonary TB was three times higher
than that of children infected with pulmonary TB. This result was
in line with a study conducted in Gondar, Ethiopia [8]. The reason might
be that the easy diagnostic technique for EPTB is not available
in most of our clinical settings, resulting in delayed initiation of
anti-TB treatment leading to rapid disease progression and easy
involvement of vital organs.
During follow-up, this study revealed that anemia was
associated with higher child death. No previous studies examined
anemia during follow-up, but at the beginning of the study, it was
identified as a predictor of mortality in studies conducted in Gondar
(Ethiopia) and Thailand [8-24]. Higher mortality with anemia may
be associated with decreased oxygen and nutrient care capacity of
the blood, resulting in inadequate oxygen and nutrient supply to
vital organs that become synergistic with TB and HIV [8]. In contrast
to other studies in Gondar (Ethiopia) [8], Thailand [24], Nigeria [6],
Malawi [26], and a single study in four developing countries [23];
WHO staging, CD4 count, and hemoglobin level at baseline were
not significantly associated with mortality in this study. The reason
might be that unlike these studies, our study assessed the effect of
the variables during follow-up time and at baseline. Most of these
variables were significantly associated during follow-up, which
shows a better effect on the outcome variable than at baseline.
This is one of the strengths of this study. Assessing the effect of
these variables during follow-up enables us to overlook the more
accurate effects of exposure variables on the outcome variable. The
study also considered the time of the event, which enables us to
consider the contribution of censored cases.
Limitation of the Study
Since the study was a retrospective review of the chart (secondary data), some variables not documented in the child’s medical records were missed. A further prospective study is needed to address other important issues not addressed by this study.
Conclusion
The mortality rate of children co-infected with TB and HIV in two zones of the Tigray region was high. Most deaths occurred within the first six months of the follow-up period. Underweight at baseline, IPT non-user, poor ART adherence, extrapulmonary TB, advanced WHO staging during follow-up, advanced/severe immunosuppression status during follow-up, and hemoglobin level < 10mg/dl during follow-up were predictors of increased mortality. This study is important for planning and decision making by pointing out gaps to make a successful strategy to combat TB and HIV and related consequences to increase the overall effectiveness of therapy in TB and HIV co-infected infected children.
For more
Articles on : https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.