The Effects of Surface Roughness on the Functionality of Ti13Nb13Zr Orthopedic Implants
Introduction
In 2034, for the first time in US history, the number of older Americans over 65 years old will exceed the number of children under 18 years old [1]. One of the health challenges in an aging nation, could be the issues of bones and joints. According to the American Academy of Orthopedic Surgeons (AAOS), by 2030, total hip and knee replacements are expected to grow up to 171% and 189%, respectively [2]. The true picture of bone and joint health in the United States is even worse if the number of civic, sport, and military incidents could be added to the current statistics. Bone is the second most transplanted tissue after the blood, and annually more than 1.6 million bone graft operations are performed in the USA, which is the highest in the world [3, 4]. The aging US population, a rise in obesity, and an inactive lifestyle complicate the treatment of bone diseases and creates a national healthcare crisis. Current methods to address bone-related issues, appear inefficient or in some cases are incompatible with the prolonged life expectancy in modem societies. Numerous studies have shown the serious limitations and complications of conventional treatments for bone diseases [5-10]. Based on a comprehensive clinical data collection on the failed hip and knee surgeries within the first year of the operation, the main causes of failure were reported as: loosening and separation of bone and implants (41.4%), excruciating pain (23%) and infection (18.4%) [11]. This research aims to determine the effects of surface roughness on the functionality of implants. In this regard, wettability, cell attachment, and mechanical properties of Ti13Nb13Zr were investigated. Based on the experimental results, a range of surface roughness is proposed that meets all requirements in terms of good wettability, high cell attachment rate, and better mechanical properties.
The Important Role of Surface Roughness
Topography is considered a major factor in the performance and life span of orthopedic and dental implants, and this fact has been supported by several studies. For instance, Lange, et al. [12], Linez-Batailon, et al. [13], Kirbs, et al. [14], and many others [15-19], believe that there is a direct relation between surface roughness and the integration of bones and implants. In this regard, the science and engineering of orthopedic implants have recently received a considerable amount of attention. The phenomenon of surface roughness has received particular attention due to emerging new technologies such as additive manufacturing (AM) methods, which are capable to fabricate individual implants with desired roughness values based on the demands of each patient. Hoffman and her colleagues [20], created roughness ranging from Ra=0.07 μm to 6 μm on commercially pure titanium (CpTi) and Ti6Al4V samples. They showed that increasing the surface roughness is in favor of better integration and can increase the chance of mechanical interlocking at the interface of bone and implant. On the other hand, Schuh, et al. [21], believe that increasing the surface roughness, is not necessarily in favor of better integration and might increase the possibility of abrasion, which eventually ends in poor bone-metal bonding. Many researchers such as Albrektsson [22], Szmukler-Moncler [23], Kieswetter [24], and Rupp [25] have suggested that the surface roughness of orthopedic implants is not a singular feature, but it is an interrelated feature that should be studied from different angles through a multidisciplinary approach. Lampin [26] and Deligianni [27] highlighted the effect of surface roughness on the first critical steps of implantation in which the blood touches the implant and, protein adsorption occurs. Rupp, et al. [28] showed that surface roughness enhances osseointegration of titanium implants and, can affect the wettability behavior. Despite the existence of some research data supporting the positive influence of surface roughness on bone and implant integration, the problem is, there is no suggested optimal range of roughness values that can provide maximum protein adsorption and cell attachment. To the best knowledge of the author, a wide range of surface roughness from Ra=0.07 μm to 100 μm [20, 29] were studied by different researchers, but still, the lack of research data on the optimum range of roughness is sensible. The existing discrepancy arises from the fact that surface roughness can play a dual role in the performance of orthopedic implants. For instance, Cooper, et al. [30] by in vitro and in vivo studies concluded that an increase in the surface roughness of commercially pure titanium (CpTi) implants improved bone integration at the interface. Similarly, Buser, et al. [31] indicated that there is a tendency for an increase of boneimplant contact due to increased surface roughness. On the other hand, some researchers, such as London [32], Novaes [33], Carlsson [34], Gotfredsen [35], and Vercaigne [36], could not confirm any meaningful relation between surface roughness and bone and metal integration. Some researchers such as, Elias [37] and Wennerberg, et al. [38, 39] believe increasing the surface roughness may cause an intolerant bone response which leads to mechanical failure. Depending on the scale of irregularities of the material surface, surface roughness can be categorized into three main groups; macro roughness (>100 μm), micro-roughness (100 nm-100 μm), and nano roughness (<100 nm), any range of roughness can influence the cell response to the implant [40]. It has shown that the microtopography can maximize the interlocking between bone and the surface of the implant [41]. Wen, et al. [42] have shown that some surface properties, such as surface roughness affect the mechanical stability of the implant-tissue interface. The mechanical properties of implants should be suited and compatible with receiving tissue surfaces. Oshida [43, 44] proposed that if the roughness could be manipulated to be in the range of 1 to 20 μm, the implant’s survival rate would be acceptable, he attributed this finding to morphological compatibility of the surface. Ratner, et al [45] and Baro, et al. [46] showed that in micron-level (roughness > 10 μm) roughness can influence the mechanical properties of the titaniumbone interface, the mechanical interlocking of the interface, and the biocompatibility of the implant. Kasemo, et al. [47] showed that surface roughness in the range of from 10 nm to 10 μm may also influence interfacial biology since it is the same order as the size of the cells and large biomolecules. Micro-roughness at this level includes materials defects, such as grain boundaries, steps and kinks, and vacancies that are active sites for adsorption, therefore influence the bonding of biomolecules to the surface of the implant [48]. Keller, et al. [49] believe micro rough surfaces promote significantly better apposition than smooth surfaces, resulting in a higher percentage of bone in contact with the implant. Micro rough surfaces may influence the mechanical properties of the interface, stress distribution, and bone remodeling. Increasing the contact area and eventually increasing the mechanical interlocking of bone to the implant, can decrease stress concentrations resulting in decreased bone resorption. Rich and Harris [50] reported that some cells exhibit rugophilia, or an affinity for rough surfaces, whereas some cells failed to readily adhere to these same surfaces. Studies by Brunette [51] and Chehrouhdi, et al. [52, 53] have demonstrated that cells tend to attach and orient themselves in the grooves with specific dimensions on the roughened surfaces. Michaels, et al. [54] and Keller, et al. [55] determined that cells are more likely to attach to rough CpTi surfaces produced by sandblasting than smoother surfaces polished with 1μm diamond paste. During the surgical procedure of implantation, the implant most likely will encounter blood. Almost instantly following contact with blood, the implant surface will be covered with plasma proteins that become adsorbed onto the surface [56, 57]. It is reported that as Ti surface exposes to the blood, it takes about 10 minutes for protein-adhesion (polymorphonuclear granulocytes) to happen on the surface. During the first week after implantation of Ti, a fluid space, which contains proteins [58-60] and other inflammatory cells, separates the implant surface from the tissue. Most cells in the fluid space are not in direct contact with the surface during the first week of the healing [61]. However, during later time intervals, macrophages are adherent to the surface of Ti [62]. Macrophages (type of cells) are active around the implant and release mediators which act as messenger cells, transmitting information about the implant surface structure and composition to the surrounding tissue [63, 64]. Implants can fail due to a couple of reasons, including failure to integrate, implant fracture, implant malposition causing damage to vital structures (such as the inferior alveolar nerve, sinus membrane, a natural tooth, or an adjacent implant), and advanced loss of bone around an integrated, loaded implant, resulting in implant mobility and/or removal. Loosening and bacterial infection (or both) are the most common risks of implantation [65-68], moreover, implants can also fail due to wear, fatigue, chemical degradation, corrosion [69, 70].
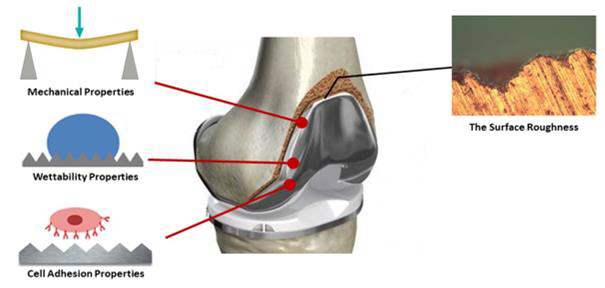
Figure 1: Surface roughness can influence surface wettability, cell interaction, and mechanical behavior of implants.
Materials and Sample Preparation
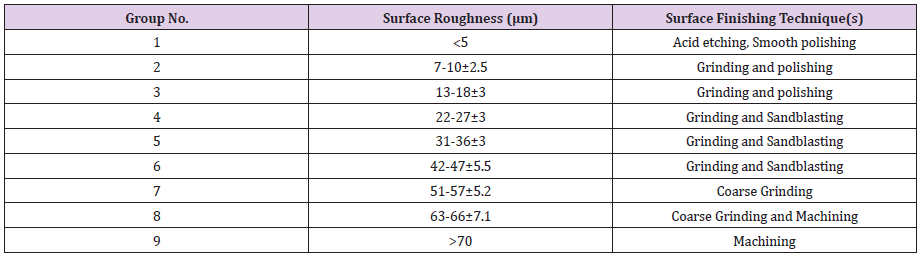
Ti13Nb13Zr (Xi’an SAITE Metal Materials Development Co., Ltd., Xi’an, China) as one of the newest members of the titanium-based alloys was studied in this research. The chemical composition was reported as; 13.0 wt.%Nb, 13.0 wt.%Zr, 0.086 wt.%O, 0.009 wt.%N, 0.0012 wt.%H, and balance Ti. Recently it has been found, Ti6Al4V releases aluminum and vanadium ions in the body, which can generate long-term health problems such as peripheral neuropathy, Osteomalacia and Alzheimer’s diseases [71,72]. Besides better compatibility, Ti13Nb13Zr has a lower Young’s modulus (E=75 GPa) than Ti6Al4V (E=114 GPa) which is a big advantage for orthopedic applications. To create desired surface roughness on Ti13Nb13Zr samples, they were subjected to different surface finishing techniques as summarized in Figure 2. The arithmetic average roughness, (Ra) was measured by the Hirox® profilometer microscope (Figure 3). Finally, nine groups of samples with different ranges of roughness were fabricated which are listed in Table 1.
Experimental Methods
The three-point bending test was performed based on ASTM E290 [73], using INSTRON 5567 universal testing machine. The point is the force intentionally was applied on the untreated side of samples and roughened side of samples were set downward. Contact angle measurement (wettability) was performed by optical tensiometer on dry and clean Ti13Nb13Zr samples for 120-150 seconds. The water droplet size was 3 to 5 μl, and glass syringe was used with 19-to-21-gauge needles. Cell culture Human MG-63 osteoblastic cells (Sigma-Aldrich, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM); Thermo Fisher Scientific, USA) with 10% fetal bovine serum (FBS) (Sigmaaldrich, USA), at 37 °C, in a 5 % CO2 humid atmosphere. DMDEM contains four-fold concentration of amino acids and vitamins. In order to perform the cell adhesion study, Ti13Nb13Zr samples were placed individually into the sterile 24-well plates and the cell suspension at a density of 5×104 cells/ml (100 μl) was pipetted onto the surface of each sample. After 4h, 500 μl complete medium was added to each well, and cells cultured for 48 hours. The samples were rinsed three times with phosphate-buffered saline (PBS) following the removal of the medium. For cell proliferation, Suspended MG-63 cells in DMEM with 10 % FCS (5 × 104 cells/0.3 mL) were seeded onto Ti13Nb13Zr samples. To prevent touching the lateral parts of the samples, Ti13Nb13Zr samples were covered by tape. After 10 min to allow cell sedimentation and adhesion to the non-adherent cells (i.e., cells that did not adhere to the surface within the given period) was then drawn up with a pipette, transferred into 12 × 75 mm test tubes, and analyzed by flow cytometry for quantitative assessment. Ti13Nb13Zr samples. To prevent touching the lateral parts of the samples, Ti13Nb13Zr samples were covered by tape. After 10 min to allow cell sedimentation and adhesion to the non-adherent cells (i.e., cells that did not adhere to the surface within the given period) was then drawn up with a pipette, transferred into 12 × 75 mm test tubes, and analyzed by flow cytometry for quantitative assessment.
Results and Discussion
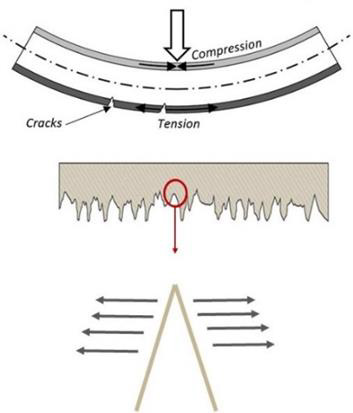
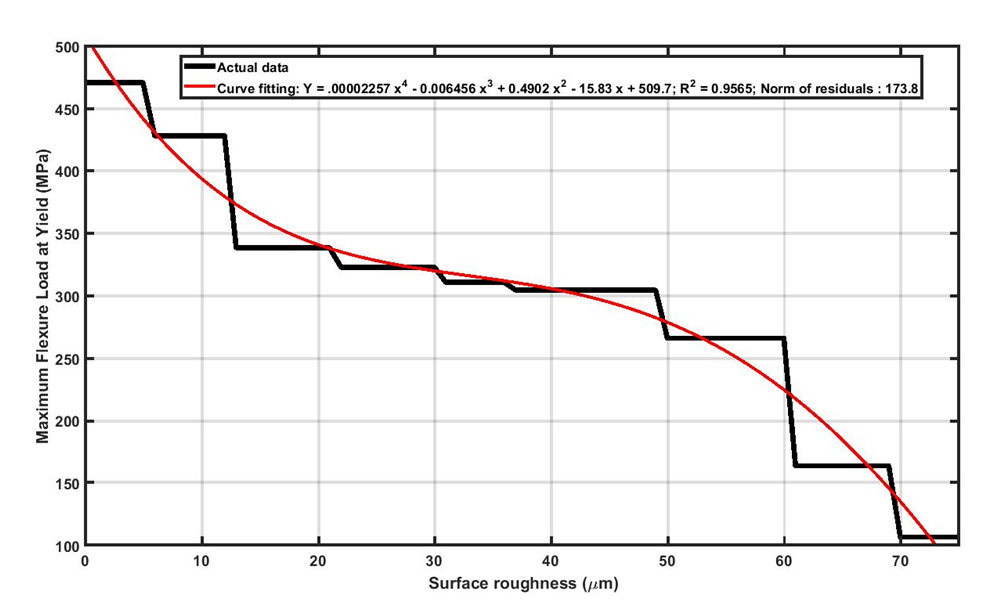
It was observed that bending test results and the failure of Ti13Nb13Zr samples are highly sensitive to the magnitude of surface roughness. This observation can be attributed to the fact that surface roughness can act as stress concertation sites (Figure 4). Deeper (i.e., sharper) scratches will convince the failure due to higher stress intensity at the tip. In fact, crack initiation and propagation happen easier on rougher surfaces. As it was seen in the bending experiments, surface integrity in rougher samples is more vulnerable and causes premature failure in Ti13Nb13Zr samples. As seen in Figure 5, the results show that the strength of Ti13Nb13Zr samples decreased obviously upon increasing the surface roughness. It should be noted that, metallic orthopedic implants must be able to carry flexural and compression stresses over 300 MPa [74]. Referring to Figure 5, it was seen that for the Ti13Nb13Zr samples with surface roughness higher than 40μm, the maximum flexural load has been decreased to less than 300 MPa. This means for Ti13Nb13Zr implants, the surface roughness should not be increased more than 40 μm. It is worth mentioning that, in this study the desired roughness values were created by subtractive methods including grinding, sand blasting and machining. The nature of the subtractive method is to remove some parts of the surface by mechanical force, which creates numerous micro-level defects as well as a high amount of residual stress on the surface. These imposed irregularities on the surface, threaten the surface integrity and eventually the stability of the sample. To fabricate rougher surfaces, more forces are applied on the samples, therefore more defects are imposed on the surface which makes them more susceptible for crack initiation.
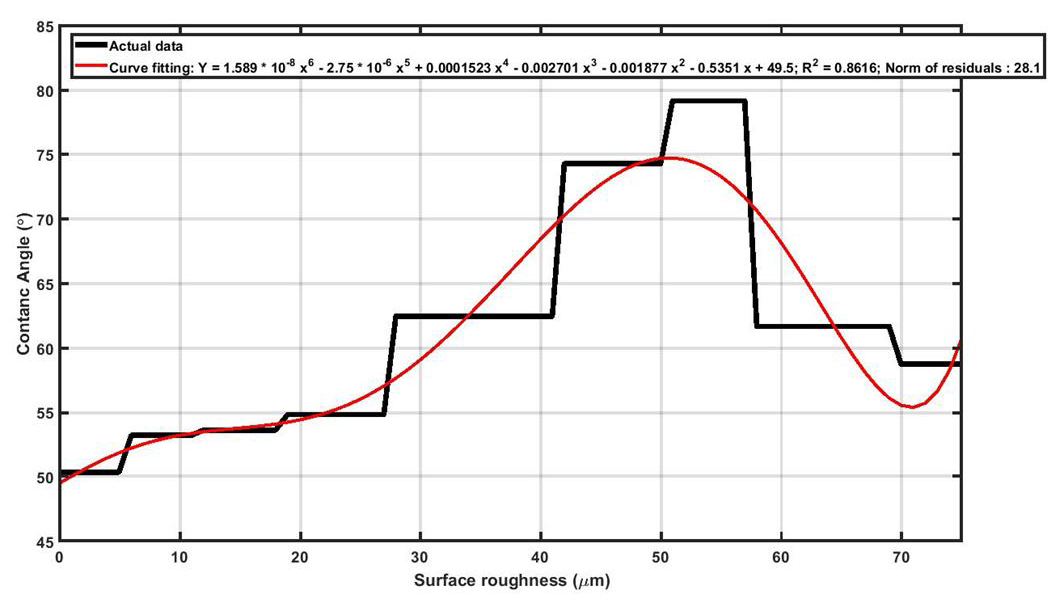
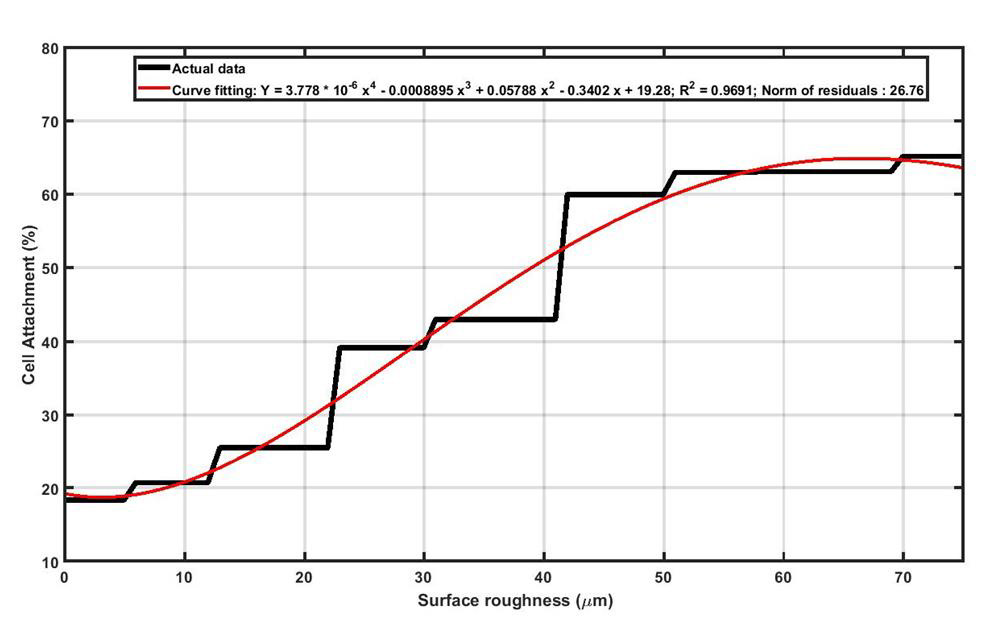
As seen in Figure 6, in wetting test it was observed that, upon increasing surface roughness of Ti13Nb13Zr samples, the magnitude of contact angle increased to a maximum value and then declined. It should be noted higher contact angle means lower wettability which is not desired for the orthopedic and dental implants. Black curve in Figure 6 shows that as long as surface roughness is approximately less than 28μm, the contact angle is almost constant for Ti13Nb13Zr samples. It can be concluded that in terms of wettability, the recommended surface roughness for Ti13Nb13Zr implants must be less than 28μm. In order to explain the increasing of contact angle upon increasing surface roughness, the “Cassie-Baxter model” can help. In fact, by increasing surface roughness, more asperities are induced on the surface and the “Air Pockets” between the grooves prevent the droplet to touch the surface and wettability decreases. Upon further increasing of roughness (60μm for Ti13Nb13Zr), the “Wenzel model” will dominants the wetting behavior. In this model, more roughness means more surface area which subsequently means higher surface energy. Higher surface energy facilities bonding between water droplet and the surface, and therefore wettability increases (lower contact angles). The cell attachment test was performed in two different segments, including cell adhesion and cell proliferation. A successful implant must provide favorable conditions for cells to adhere on its surface and support the spread and growth of the cells. As it can be seen in Figure 7, surface roughness can endorse the initial cell adhesion and, as surface roughness increases, the percentage of cells that could successfully attach to Ti13Nb13Zr samples increases. In fact, rougher surfaces can provide more preferred sites for the accommodation of cells which means cell adhesion is intensified.
Figure 5: Reduction in maximum flexural load at yielding for Ti13Nb13Zr samples with increasing the surface roughness.
Figure 7: Increasing the surface roughness of the Ti13Nb13Zr sample provides more suitable sites for cell attachment.
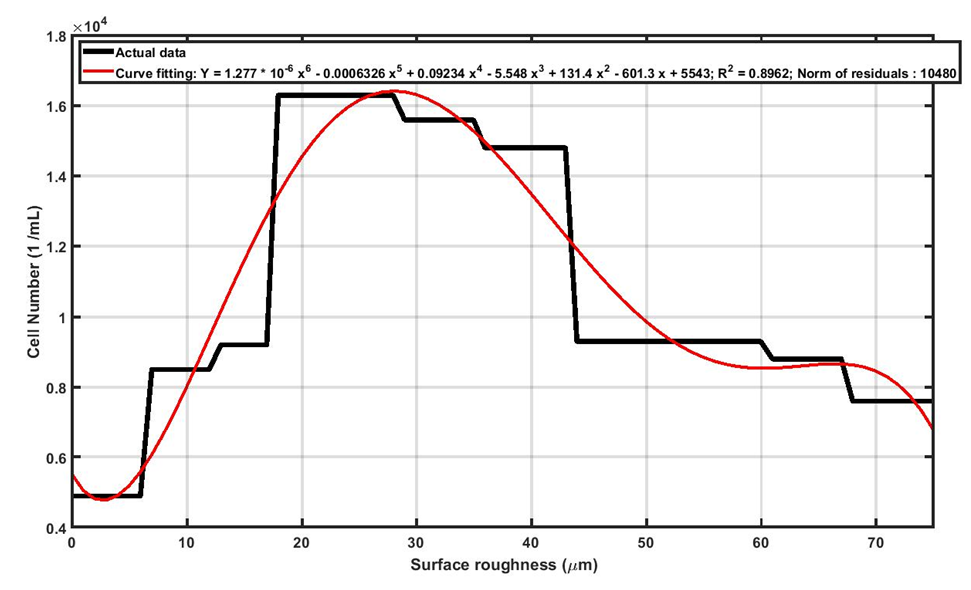
Figure 8: Increasing the surface roughness of Ti13Nb13Zr samples supports the cell proliferation to a particular level and then adversely affects it.
The mechanism of cell proliferation is different with cell adhesion. As seen in Figure 8, by increasing the surface roughness, the number of proliferated cells on Ti13Nb13Zr samples increase to a maximum value, and then declines. As the curve shows the cell proliferation is maximum around 28μm which is desirable for orthopedic implants. The surface roughness plays a dual role in cell proliferation. In other words, increasing surface roughness of Ti13Nb13Zr supports cell proliferation, but further increasing of roughness has adverse effects on cell proliferation. We will use the concept of “shelter vs. jail” to explain the dual effects of roughness on cell proliferation. First, the surface roughness can provide sites for cell to grow and get connected (shelter). Within this shelter the cells can grow and connect better than smooth surfaces. If the magnitude of surface roughness increased from certain point, the roughness acts such as barriers against the cell growth and cell connections (jail).
Summary and Conclusion
In orthopedic implants, an ideal surface, not only should play a substantial role to absorb nutrients and cells on itself, but it should actively resist any crack formation. Increasing the surface roughness in favor of more cell attraction, will increase the stress concentration sites which, eventually causes crack formation. In fact, by creating roughness on the surface of a sample, the ductility and bending strength of the specimen will be influenced. The results of this study on Ti13Nb13Zr with different surface roughness values showed that surface roughness between 20 μm to 25 μm can satisfy the major topographical requirements of a metallic orthopedic implant including cell adhesion and proliferation, bending strength, and wettability. Fulfillment of these requirements will guarantee the better functionality of orthopedic implants inside the body.
For more Articles on : https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.