in vitro Introduction, Proliferation and Medium-Term Establishment of Various Yams’ Species (Dioscorea Spp.)
Introduction
There exist about 600 species of yams (Dioscorea spp.) in the world belonging to 23 sections [1]. Some of the species have been cultivated as food crops for centuries because of the high carbohydrate content in their tubers and some others have been used as medicinal plants due to the presence of chemical compounds (mainly diosgenin) which have been used to produce steroid drugs. The main food crop species are vegetatively propagated; therefore, preservation of yams can be accomplished through in vitro short and medium-term conservation and cryopreservation.
Successful introduction of yams into in vitro culture followed by their multiplication would provide sufficient explants for various needs including experiments on germplasm preservation. Introducing yams into in vitro culture has been done using various explants such as shoot and node culture, seed culture (in case of wild species) as well as tuber culture using various culture media [2-7]. Information on multiplication rates of various accessions is important for their maintenance and other practical uses. It is also desirable that all genetic potential of the preserved germplasm, including multiplication rate, be maintained or stable in the long run. However, reports have indicated the occurrences of genetic changes resulting in soma-clonal variation in the in vitro culture (see Scowcroft, 1984) [8]. Evaluation of long-term multiplication rates is, therefore, important to ensure that the in vitro cultural practices applied do not alter multiplication rate of the materials maintained. More than 70 accessions of yams have been maintained in vitro in the in vitro culture and cryopreservation laboratory, genebank department of the Institute for Plant Genetics and Crop Plant Research (IPK) at Gatersleben. Experiments reported in this paper are aimed at
a) Introducing yams into in vitro culture using aerial tubers as well as assessing the effect of different culture media on the success of in vitro introduction,
b) Determining multiplication rates of various yams accessions and
c) Determining the medium-term multiplication rate of five selected yam genotypes.
Materials & Methods
in vitro Introduction
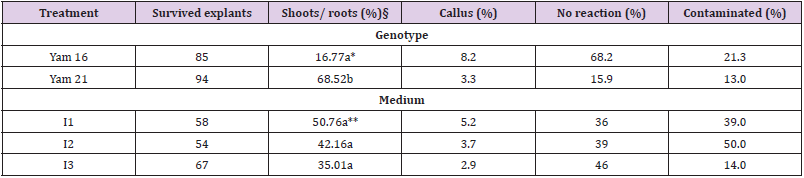
Experiment on the in vitro introduction was conducted during February and March 2001 using aerial tubers of two different yam accessions: Yam 16, confirmed as D. bulbifera L. obtained from Botanic Garden of the University of Frankfurt, Germany, and Yam 21, identified as D. polystachya Turcz. (Previously indicated as D. oppositifolia L.) obtained from Botanic Garden of the University of Padua, Italy. Selected small aerial tubers of Yam 16 (diameter of 1-2 cm) and the aerial tubers of Yam 21 (diameter about 1 cm) were harvested from 9 months old plants (planted in March, harvested at the end of December 2000) grown in pots in the greenhouse. Tubers were washed with water and then with 70% alcohol before being sterilized using 20% sodium hypochlorite plus 2 drops of Tween 20 on a shaker for 15 minutes. The tubers were than washed four times with sterile water before the introduction into culture. Samples of Yam 21 were mostly cultured as an entire tuber (cut into two only when necessary) while many of the tubers of Yam 16 were cut longitudinally into two (head is divided into two parts) before being cultivated in 15 ml ‘introduction’ medium in SIGMA glass culture tubes (length 15 cm, diameter 2.5 cm). Three different media were tested, namely I1 [9] salts + 0.1 mg/l thiamine + 2 mg/l indole acetic acid [IAA] + 5 mg/l kinetin), I2 (MS salts + 0.1 mg/l thiamine + 2 mg/l IAA + 10 mg/l kinetin), and I3 (MS salts + MS vitamins + 0.2 mg/l α-naphthylacetic acid [NAA] + 0.5 mg/l 6-benzylaminopurine [BAP]). All media used 3% sucrose, 1% agar and 0.2 % activated charcoal (AC). Each treatment combination was replicated two times by using two culture racks each consisting of 18 culture tubes. The two racks of each treatment combination were placed on different shelves in the growth chamber with 16h/8h light/dark period using fluorescent lamps, 25°C and light intensity of 60-80 μmol.cm-2. s-1. Observation was conducted during four weeks on the percentage of shoot and roots production, callus formation and the explants which showed no reaction as well as infected explants (infected explants were directly discarded from the growth chamber and were not used in calculating the percentage of each parameter). Percentages of the explants produced only roots as well as roots and callus were also noted, however, were not presented in the results due to their very small number.
Multiplication Rate
Observation on multiplication rates involved 46 clones comprising 39 accessions and 18 different species of a research collection maintained in IPK. The materials were obtained from various sources such as botanic gardens or brought by students from other countries either as in vitro plantlets, tubers or seeds. Most of the genotypes were introduced into culture via in vitro sowing as well as using nodal explants of plants grown in the greenhouse. Most of the collections have been maintained since December 1999 by means of sub-culturing single nodal explants (with one small leaf) every two months in 15 ml of MS medium + 0.1 mg/l NAA + 2 mg/l BAP with 3% sucrose, 1% agar and 0.2% AC in SIGMA glass culture tubes (length 15 cm, diameter 2.5 cm). Previously, culture environment used was a 25 °C growth room with 16 h photoperiod (8 h dark) and 60-80 μmol.cm-2.s-1 light intensity using fluorescent lamps. However, since August 2000, when the explants number of each accession and the number of accessions increased, the collections were divided into two different groups cultivated either at 20 °C or at 25 °C with maximum number of 18 explants per in vitro clone. This division was based mainly on the environmental condition from which each accession was geographically originated and distributed, and the features of the plantlets. The study on Yam 54-1 (D. alata L.) and Yam 58-1 (D. cayenensis Lam.) was started at the 8th cycles of sub-culture. Yam 64-1 (D. esculenta L.) and Yam 73-1 (D. esculenta L.) were introduced into in vitro culture in April 2002 and the study was carried out at the first cycle of sub-culture after the number of plantlets reaches full rack (18 plantlets). For the other genotypes, the study was initiated after 3 cycles of subculture. Two terms of multiplication rate were calculated, namely theoretical and practical multiplication rates. Theoretical multiplication rate (TMR) defined as number of total explants possibly obtained divided by number of survived explants while practical multiplication rate (PMR) defined as number of total explants possibly obtained divided by the original number of explants at the beginning of the subculture. An explant was defined as a single node including one leaf taken from any part of the plantlet (excluding the original node). The explants for each sub-culture were sampled randomly out of 18 collected plantlets of the previous sub-culture to meet 18 plantlets requirement for the next generation. Three consecutive measurements were obtained for each group.
Medium-Term Multiplication Rate
Five genotypes representing 4 species namely Yam 16, Yam 21, Yam 26 (D. polystachya Turcz), Yam 54 and Yam 58 were selected for the evaluation of medium-term TMR by continuing bimonthly subculture up to 10 cycles (20 Months). The medium, culture tube and the culture environments applied were similar to that of maintenance culture. As well, sampling and sub-culturing practices were similar to the method described previously. The study started after 8th cycle of subculture.
Statistical Analysis
Percentage of shoots and roots production of the experiment on in vitro introduction was subjected to ANOVA to determine the main effect and the interaction between medium and genotype. The t-test was used to assess the differences on TMR and PMR mean values among all genotypes as well as between TMR and PMR at each genotype. For study on the medium-term TMR, Pearson correlation coefficient was used to assess the relationship be-tween variables (TMR and number of sub-culture) on each genotype. Furthermore, polynomial regression analysis was implemented to estimate the curve, which most fit to the trend of the data. All the data were tested for normality distribution and equal variance before analysis. The analyses were conducted with the help of SigmaStat software, Version 2.0, SPSS Inc., Chicago.
Results
in vitro Introduction
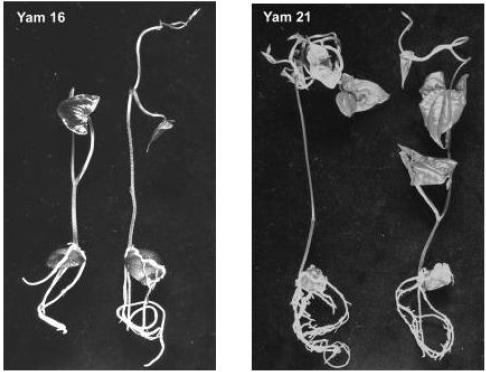
Germinated explants were on various stages of development after four weeks of cultivation. The latest stage of development for the two species are given in Figure 1 (taken 5 weeks after culture). ANOVA was conducted only for the formation of shoots and roots (germinated explants) since they were considered to be the most important developmental character for the in vitro introduction. The statistical analysis indicated that there was no interaction between genotype and ‘introduction medium’ used. The main effects of each treatment, therefore, discussed.
Figure 1:Latest morphological developmental stages of Yam 16 (D. bulbifera L.) and Yam 21 (D. polystachya L.) after 5 weeks of in vitro introduction using aerial tubers.
Genotype effect: The fact that Yam 16 was able to produce shoots and roots in this experi-ment (Table 1) is encouraging, since in our previous work of in vitro introduction no shoots and roots were obtained. In these studies (unpublished), bigger tubers (about 5 cm in diame-ter) of Yam 16 and Yam 22 (also D. bulbifera L.) were cut into sets of about 2x1.5 cm skin area and 1 cm thickness. Only callus (87.2% and 40.5% for Yam 16 and Yam 22, respectively) was produced and many explants were contaminated. In the present experiment, Yam 21 germinated faster and produced significantly higher shoots and roots compared to Yam 16 (Table 1). Special morphological characteristics of tuber seemed to be one of the factors re-sponsible for these differences. D. bulbifera has a trait of a smooth tuber surface while the tuber of D. polystachya has many papillae on its surface from which germination can take place, although, in most cases, germination occurs on the head of the tuber. In the previous in vitro introduction using Yam 25 (D. sansibarensis Pax) which also possesses many papillas on the tuber surface, we were able to obtain 28% germination (shoots and roots) (un-published). Genotype influences on in vitro introduction have also been indicated by Ma-laurie et al. (1993) [5] on their in vitro introduction using seeds and nodal cuttings.
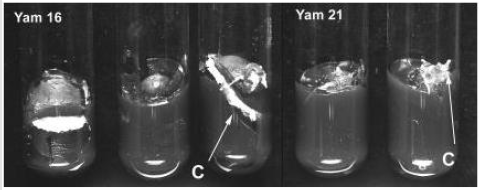
Contamination level was generally lower compared to that of underground tubers of D. trifi-da, D. rotundata and D. cayenensis reported by Mitchell et al. (1995a) [3] who obtained an average contamination of 62% (with skin) and 32% (without skin), even though the explants were double sterilized. Contamination of the explants was higher (21.3 %) on Yam 16 com-pared to that of Yam 21 (13.0%) (Table 1). Cutting of the tuber might have increased the vulnerability of Yam 16 to contamination. The same reason also explains the higher callus formation on Yam 16, since callus formed in this accession was wound callus, produced on the border of the cutting surface, while callus formation on Yam 21 occurs on papilla on the tuber surface (Figure 2). Higher percentages of Yam 16 explants did not show any response (68.2%) compared to that of Yam 21 (15.9%) after 4 weeks of culture.
Figure 2:Callus (C) formation on Yam 16 (D. bulbifera L.) and Yam 21 (D. polystachya L.) explants from aerial tubers.
Table 1: Effects of genotype and culture medium on the in vitro development of yams 4 weeks after culture$.
Note: Contaminated explants were not included in calculating percentages of different developmental characters. §) Parameter analysed statistically. *) Numbers within the column below genotype followed by similar letter are not significantly different at the 5% level based on F-Test. **) Numbers within the column below medium followed by similar letter are not significantly different at the 5% level based on the F-Test.
Medium Effect
Although medium I1 showed slightly better effect (50.8%) on the production of shoots and roots compared to that of I2 (42.2%) and I3 (35.0%), this effect was statistically not significant (Table 1). Increasing concentration of kinetin from 5 mg/l (I1) to 10 mg/l (M2) on MS salt medium did not increase the germination percentage of in vitro introduced aerial tubers. This finding was in agreement with that reported by Zok et al. (1998) [6] who obtained high numbers of shoots with kinetin concentration ranging from 5 to 10 mg/l on in vitro introduction of D. alata L., D. esculenta (Lour.) Burk and D. rotundata Poir using nodal explants. Furthermore, supplementing MS medium (plus vitamins) with 0.2 mg/l NAA and 0.5 mg/l BAP (M3) instead of supplementing pure MS salt medium with IAA and kinetin (in I1 and I2) did not increase the percentage of aerial tubers’ germination. I3 medium (with BAP and NAA) was used by Nair and Chandrababu (1994) [7] to introduce D. alata L., D. esculenta (Lour.) Burk and D. rotundata Poir using nodal segments. Malaurie et al. (1993) [5] used 1 mg/l NAA and 0.2 mg/l BAP to replace activated charcoal and glutamin in their maintenance medium (basal culture medium containing Knop’s modified mineral nutrients, MS modified vitamins, 3% sucrose and 0.8% agar, 0.2% activated charcoal and 200 mg/l glutamin) as special ‘introduction medium’ for clones which were difficult to be introduced using maintenance medium, while Mitchell et al. (1995a) obtained high survival rates of meristem tips (83%) for D. trifida ‘Yampie’ using MS salts supplemented with 0.2 mg/l BAP and 1.0 mg/l NAA. Medium I2 (MS salts with 10 mg/l kinetin) exhibited the highest percentage of contamination (50%) followed by I1 (39.0%) and I3 (14.0%). Callus formation was generally low, although there were small differences on callus formation among the three media used. The percentage of non-responsive explants was slightly higher in medium I3 than in the other two media (Table 1).
Short-Term Multiplication Rates
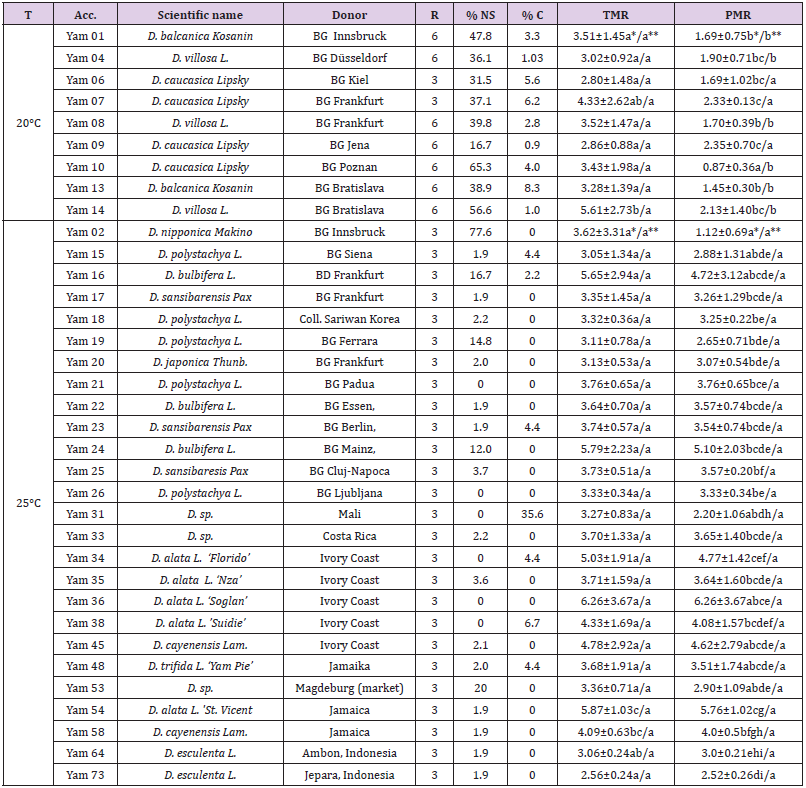
It has been indicated that grouping the accessions in two different cultivation temperatures was based mainly on their geographical origin. Those cultivated in temperature of 20°C are originated and distributed in sub-tropical and temperate areas such as North America (Atlantic Yam - D. villosa L.) and Southeastern Europe (D. balcanica Kosanin; D. caucasica Lipsky) and used as medicinal plants. These species possess morphological characteristics of smaller stem diameter and smaller leaf size compared to those cultivated in 25 °C which originated and distributed in tropical areas of the Asian continent (Chinese Yam - D. poly-stachya, Greater Yam - D. alata, Potato Yam - D. bulbifera), the African continent (D. sansibarensis, D. bulbifera, D. alata, D. cayenensis-rotundata) and Central and Northern South America (Cushcush Yam - D. trifida, Guinea Yam - D. cayenensis-rotundata), and cultivated mainly as food crops, even though, some of them are toxic and some others are used for medicinal purposes [10]. An exception in our cultivation was that Yam 2 (D. nipponica Makino) which is distributed in Japan, Korea, northern and central China, former USSR and possesses similar morphological characteristics as those cultivated in 20°C, was cultivated in 25°C. This accession was used as a comparison to the tropical species. Al-most all of the accessions of sub-tropical areas were introduced into in vitro culture with more than one clone, and four of these clones were lost in course of the maintenance. For some accessions, two clones were used in calculating multiplication rates. The second intro-duction of these accessions was considered as replication (Table 2).
Table 2: Multiplication rate of various yams (Dioscorea spp.) cultivated in two different temperature regimes about 2 months after transfer.
Note: Numbers (values±standard errors) within the column (between accessions) followed by the same letter (s) are not significantly different at α=0.05 based on t-test. **) Numbers (values±standard errors) within the row (within accession) followed by the same letter (s) are not significantly different at α =0.05 based on t-tes, BG = Botanical garden, C= contaminated explants, NS = Not survived explants, TMR = theoretical multiplication rate, PMR = practical multiplication rate, R = replication, T = temperature.
Accessions of the 20°C Group (Temperate and Sub- Tropical Yams):
Theoretically yams accessions, cultivated at 20°C, were able to multiply 2.8 ±1.48 to 5.61± 2.73 times (Table 2.). Yam 14 (D. villosa) has the highest multiplication rate and was statistically different compared to other accessions except to Yam 07 (D. caucasica). Other accessions were statistically not significantly different among each other. In practice, however, only rates of 0.87±0.36 to 2.35±0.70 were able to be obtained. The lowest PMR was exhibited by Yam 10 (D. caucasica) which was significantly different compared to the other genotypes. The highest PMR was shown by Yam 09 (D. caucasica), but this was not significantly different from that of Yam 04 (D. villosa), Yam 06 (D. caucasica), Yam 07 (D. caucasica), and Yam 14 (D. villosa) (Table 2). High percentages of not survived explants exhibited by Yam 10 seemed to be responsible for the lower in vitro PMR compared to other accessions of similar species and even different species (Table 2). In six of nine accessions of temperate and sub-tropical yams, TMR was significantly higher than PMR. Only on three accessions, all of D. caucasica, TMR was similar to PMR, which was mainly due to lower percentages of not survived and/or contaminated explants exhibited by these three accessions (Table 2). Percentages of not survived explants of accessions cultivated in this temperature regime were generally high (16.7% -56.6%) which indicated that not all explants from the temperate and sub-tropical yams can be used as material for propagation.
Accessions of the 25 °C Group (Tropical Yams)
TMR of yams grown at 25 °C ranged from 3.05±1.34 to 6.26±3.76 (Table 2). The lowest value exhibited by Yam 73 (D. esculenta) and the highest by Yam 36 (D. alata ‘Soglan’). In general, higher TMR was exhibited by D. alata (Yam 34, Yam 35, Yam 36, Yam 38, Yam 54), D. bulbifera (Yam 16, Yam 22, Yam 24), and D. cayenensis (Yam 45, Yam 58) and lower TMR was shown by D. polystachya (Yam 15, Yam 18, Yam 19, Yam 21, Yam 26) and D. esculenta (Yam 64, Yam 73). Statistically, however, only two genotypes (Yam 54 - D. alata - and Yam 58 - D. cayenensis) showed significant difference than the others. No significant difference of TMR was observed among other genotypes.
Practically, only factors of 1.12±0.69 to 6.26±3.67 times can be produced by accessions grown in this temperature. However, the lowest value belongs to Yam 02 (D. nipponica Makino) which is actually an accession of sub-tropical area and showed high percentages of not survived explants (77.6%). This accession was significantly lower in PMR than most of accessions cultivated in the same temperature regime. Other genotypes exhibited similar PMR among each other except that Yam 54 exhibited a significantly higher PMR than that of Yam 73 (D. esculenta), Yam 64 (D. esculenta), Yam 53 (D. sp.), Yam 31 (D. sp.), Yam 26 (D. polystachya), Yam 25 (D. sansibarensis), Yam 20, Yam 19 (D. polystachya), Yam 18 (D. polystachya) and Yam 15 (D. polystachya). In addition, Yam 34 (D. alata) showed significantly higher PMR than that of Yam 31 (Dioscorea sp.), Yam 53 (Dioscorea sp.) and Yam 73 (D. esculenta). As well, the PMR of Yam 25 (D. sansibarensis) was significantly higher than that of Yam 64 and Yam 73 (both D. esculenta), and Yam 26 (D. polystachya) showed significantly higher PMR than that of Yam 73. The reason for these differences were the high percentages of contaminated (Yam 31 - 36% -) and not survived explants (Yam 53 - 20% -), in addition to lower TMR in combination with several not survived explants (Yam 64 and Yam 73).
Medium-Term Multiplication Rate
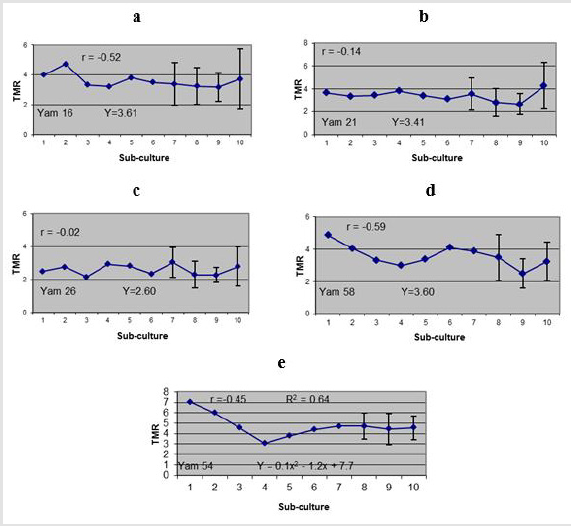
Generally, correlation coefficient (r) indicated a decrease in TMR with the increase in number of sub-culture (negative correlations). A slightly higher correlation was exhibited on Yam 16 and Yam 58 (r=-0.52 and r=-0.59 respectively (Figure 3a- 3e). However, no significant difference of r was observed in all genotypes tested. Polynomial regression analysis indicated that neither linier nor quadratic regressions were significantly fit the trend of the data for Yam 16, Yam 21, Yam 26 and Yam 58 (Figure 3a-3d respectively) even though probability of linear regression was slightly higher (low p value) for Yam 16 (R2 = 0.2, p=0.12) and Yam 58 (R2=0.34, p=0.08) in comparison to that of quadratic regression. Coefficient determination (R2) for Yam 21 and Yam 26 were very small with the probability of quadratic regression (R2 = 0.12, p= 0.51 and R2 = 0.02, p=0.82, respectively) being slightly higher than that of linear regression. It was concluded, therefore, that the TMR of the four genotypes did not changed (constant) with the increase in number of subcultures. For Yam 54, on the other hand, coefficient determination for quadratic regression showed significant difference (R2=0.64, p=0.04). The TMR decreased from the first cycle up to the fourth cycle than increase slightly up to the tenth cycle of sub-culture (Figure 3e).
Figure 3:Medium-term MR of five Yams’ genotypes after 10 cycles of bi-monthly sub-cultured; a. Yam 16 (D. bulbifera), b. Yam 12 (D. polystachya), c. Yam 26 (D. polystachya), d. Yam 58 (D. cayenensis), e. Yam 54 (D. alata). Bars indicated standard errors (calculated only at several points) based on 18 samples.
Discussion
Traditionally, genetic conservation of vegetatively propagated crops including Dioscorea spp. has been done through field cultivation. Field maintenance, however, cannot guarantee the security of collection, since the loss can occur through pests and diseases, virus, poor sprouting, unfavorable storage conditions, drought and poor handling, which has been reported for yams to achieve 10% annually [11]. Beside this, it is also time and space consuming and laborious. in vitro gene bank, therefore, offers a better way of conserving genetic diversity of such crops. To be able to conserve yams germplasm through in vitro culture using slow growth conditions and cryopreservation, as well as for other purposes such as rapid multiplication, producing virus free plantlets through meristem culture, and germplasm exchange, in vitro introduction would be the first step. This has to be followed by the establishment of the cultures for which information on the multiplication rates of the introduced clones is important.
Previous research has shown that in vitro sowing gave the highest rate of success followed by introduction of nodal cuttings [5]. But yam (Dioscorea spp.) does not always produce seeds in the field [12]. Meristem tips have also been used with high success as primary explants. It takes, however, slightly longer periods (28 weeks) to produce plantlets [3]. Attempts to introduce yams using underground tubers have not been successfully done, since all the cultures became necrotic within eight weeks of culture [3]. Our experiment indicated that yam can be introduced into in vitro culture by means of aerial tuber in a quite short time and that cultivating whole tuber of small size would be more suitable than sets of cut tuber, which may result in high contamination and callus production.
The experiment also evaluated three different culture media which have been used for introduction and establishment (with slight modification). In I1 and I2, different concentrations of kinetin combined with IAA were used to supplement MS basal medium [6], while in I3, BAP and NAA were used to supplement MS medium [7]. The results indicated that there was no statistical difference between these three media, although I1 medium gave slightly greater effect, followed by I2 and I3, respectively. This finding was in contrast with that of Mitchell et al. (1995a) [3], who reported that D. cayenensis, D. trifida and D. rotundata were more responsive to BAP than to kinetin for shoot production. This may, however, be explained by lower concentrations of BAP and NAA used in our case. Furthermore, the species and materials used in these studies were different.
Information on multiplication rates is not only important for commercial purposes of rapid multiplication. It is also useful to support decisions of whether certain measurements should be taken in case of a given accession for its safe germplasm preservation. Theoretical multiplication rate represents genetic potential of the accession to reproduce in vitro in the next subculture. Practical multiplication rate, on the other hand, shows environmental influences on the genetic potential which in this case including selection of the explants, culture media used, sterility of culture environment and the technical work of sub-culturing.
Several studies have been conducted on multiplication rates of yams [3,4,6]. Those studies, however, were focused only on some edible, tropical yams and on the theoretical multiplication rate. TMR shown by D. cayenensis (Yam 45) in our experiment was higher (4.78±2.92) than the multiplication rate of nodal explants of the cultivar RLYY of the same species grown in various establishment media, even after 12 weeks of sub culture (maximum of 4.3 for basal medium with 0.5 mg/l BAP and 0.05 mg/l NAA), reported by Mitchell et al. (1995b) [4]. For D. trifida ‘Short Neck Yampie’, however, they were able to obtain a multiplication rate of 5.0 after 4 weeks of subculture on basal medium supplemented with 0.5 mg/l BAP using nodal explants initiated from small tubers which was higher than our finding on D. trifida ‘Yampie’ of 3.68±1.91 (Table 2). These differences may be attributable to different media used and different methods of determining the MR (TMR). Mitchell et al. (1995b) defined MR as summation of original node, new nodes and shoots, while we used only new nodes and shoots.
All accessions cultured at 25 °C showed similar TMR and PMR indicating that tropical yams with more succulent stem can be propagated in vitro using nodal explants from any part of the plant. Mitchell et al. (1995b) used explants of various origins in D. cayenensis and D. trifida. They found that older nodes from plantlets grown in vitro, regardless of their origin, produced more shoots. Younger nodes grew weaker, but there was more swelling and bud development on nodal regions which, in the next subculture, resulted in higher growth. For the sub-tropical/temperate yams possessing more woody stem, however, not all explants can be propagated upon culture.
Monitoring genetic stability during in vitro germplasm maintenance is considered important due to the possible genetic abnormalities affecting the trueness-to type of the preserved materials. This has been implemented in various in vitro gene banks in the world by evaluating morphological, cytologycal, biochemical and DNA (RNA) characteristics [13-15]. In the context of further studies in yams germplasm conservation including cryopreservation, in which a great number of explants are needed, consistent production of number of shoots, nodes and branches (proliferation) enable a determination of an efficient number of plantlets to be maintained and/or multiplied for a continuously running experiment.
Four of the five genotypes (Yam 16 - D. bulbifera -, Yam 21 and yam 26 - D. polystachya -, and Yam 58 - D. cayenensis) used in this study showed a constant TMR up to ten cycles of culture since the initiation of the study. Yam 54 (D. alata) initially showed reduction of TMR but started to recover after 4 cycles of sub-culture. The high fluctuation of TMR for Yam 54 is, therefore, only an epigenetic effect which might be attributable to its sensitivity to certain environmental changes including medium (which was newly prepared at each cycle), explants selection or other practical works of tissue culture.
Conclusion
This study demonstrated that aerial tubers of yams (Dioscorea spp) can be used as source materials to be introduced into in vitro culture. Yams originated and distributed in sub-tropical and temperate areas are able to produce almost as many explants as tropical yams. However, not all of these explants can reproduce in the next subculture on the culture medium used for this study. For these accessions, therefore, care should be taken on the selection of explants, and further research should be done aiming at improving medium and other conditions for their multiplication. For tropical yams, on the other hand, every node explants regardless of their position is able to reproduce upon subculture. TMR was consistent after 10 cycles of sub-culture on four of five genotypes studied; one genotype showed reduction of TMR at initial cycles of sub-culture but recovered after 4 cycles.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.