Investigating Reliable Recovery Biomarkers of Ischemic Stroke: An Evidence-Based Study
Introduction
According to the World Health Organization, 15 million people worldwide suffer a stroke each year. Of these, nearly five million die and another five million are left permanently disabled [1]. Millions of stroke survivors are left with very limited motor functions or complete paralysis and depend on assistance [2]. Poststroke survivors majorly suffer from motor, sensory, cognition and language impairments. These impairments occur due to the loss of functions of brain regions surrounding the area of injury due to lesions which further lead to death of neurons of those regions [3]. Majority of cases of stroke are ischemic stroke [4]. Stroke recovery is the complex process due to its heterogeneous nature, where the making choices of treatment and prediction of outcomes and treatment responses are difficult [5].Prediction of outcomes in stroke can be useful to determine which intervention (such as behavioural and therapeutic interventions) will be more effective in stroke patients during the rehabilitation.
However, the methods for accurate prediction of longterm outcomes would allow clinical trials of restorative and rehabilitation interventions to be stratified based on the potential for neurobiological recovery in a way that is not possible if trials are performed in the absence of valid biomarkers [5,6]. A Stroke Recovery Biomarker (SRB) can be defined as an indicator of disease state that can be used as a measure of underlying molecular or cellular processes that may be difficult to measure directly in humans, and could be used to understand the outcome, or predict the recovery or treatment response [5]. Thus, when dealing with a condition as heterogeneous as stroke, validated biomarkers of recovery could help plan treatments and support efficient allocation of resource while maximizing outcome for the patients [7]. Identification of recovery biomarkers shall assist to advance the practice, rehabilitation and recovery after stroke. This systematic review has assessed the role of biomarkers and predictors of recovery in ischemic stroke.
Material and Methods
This systematic review was designed as per the guidelines of Preferred Reporting Items for Systematic reviews and Meta- Analysis (PRISMA) [8-10] and registered with PROSPERO (CRD42020209833) on 18th October 2020 [11].
Eligibility Criteria
Case-control studies reporting the biomarkers and predictors of recovery in ischemic stroke patients in English language published after the year 2000 were included. Studies on animal models, including diagnostic markers, prognostic markers and patients with other psychiatric or neurological conditions (other than stroke) were excluded.
The PICO (Population, Intervention, Comparator, and Outcome) format for this systematic review is:
• P: Patients diagnosed with ischemic stroke and no other psychiatric or neurological condition (other than stroke).
• I: Biomarkers or predictors of recovery in ischemic stroke.
• C: Relative levels of recovery biomarkers in post ischemic stroke patients were compared with the levels of recovery biomarkers found in control subjects.
• O: The most important outcome is the recovery of patients with ischemic stroke. The extent of recovery in ischemic stroke patients is mainly in terms of motor recovery, cognition recovery, sensory and language recovery. Recovery outcomes were measured using the standardized mean difference in level of biomarkers for individual studies.
Information Source
A systematic literature search was conducted using PubMed, Wiley online library, Rehab Data and PEDro Database. First, a comprehensive search was performed individually for all components of PICO. Later individual searches were combined with the help of Boolean operators (AND, OR, NOT). Search was performed with a combination of MeSH (Medical Subject heading terms) and keywords terms. The MeSH terms searched were “stroke”, “Rehabilitation”, “Biomarker” and “Brain Infarction”. Search was performed in September 2020 and was also updated before the submission. Search strategy was developed as per the Cochrane checklist of developing search strategy. [12] Structured strategy for PubMed is given in the Appendix A1.
Study Selection
Studies obtained through initial search from different databases were combined after removing duplicates using the Endnote “find duplicates” filter. A single reader read the title and abstract of all the obtained references. Screening of search records was performed against the predefined inclusion criteria and irrelevant studies were excluded. The reasons for excluding the studies during screening were also documented. Once preliminary articles were identified as potentially eligible, the papers were fully reviewed by two members to determine inclusion; and, disagreements in between were resolved by consensus. In case of multiple publications of the same study, the most recent publication was considered. Bibliographies and citation sections of retrieved articles had been reviewed for additional pertinent studies.
Data Extraction
A pre-specified data collection template was used to extract data from the articles obtained after screening and all disagreements were resolved by discussion among team members. The following information was extracted from the potentially eligible full-text studies;
• Publication details: Author’s name, year, journal’s name, country.
• Population related details: Total enrolled participants, gender, age, days post stroke, stage of stroke, number of participants in case-control groups.
• Intervention related details: Name of biomarker or predictor, type of biomarker or predictor, method to recognize biomarker or predictor, levels of biomarker or predictor in case-control groups.
• Outcome related details: Type of recovery, recovery in casecontrol groups, tools for assessing recovery, National Institute of Health Stroke Scale (NIHSS) score, Modified Ranklin Scale (MRS) score, correlation values in between biomarker/ predictor and recovery.
Quality Assessment
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of studies independently by two authors. The NOS consists of three domains: selection, comparability, and outcome/exposure. It assigns a maximum of four stars for the selection, two stars for comparability and three stars for the exposure category. Thus, nine stars altogether indicate the high quality, seven to eight stars indicate medium quality and six or less stars indicate low quality. Any conflicts were resolved by consensus and individual score of each study was recorded which represents the quality of study.
Risk of Bias in Individual Study
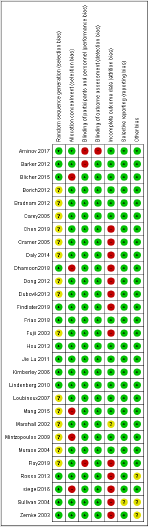
The Cochrane Collaboration’s tool for assessing the risk of bias was used for the risk-bias-assessment at the study level. The assessment was done for the domains namely random sequence generation and allocation concealment for selection bias; incomplete outcome data (attrition bias); selective reporting of outcome (reporting bias); and other biases including publication bias [12].
Data Synthesis and Analysis
The extraction of data from eligible studies was done in Excel spreadsheet, Microsoft Office 2010 (Washington, USA). A meta-analysis of studies was performed through standardized mean difference of recovery biomarker levels. The mean levels of recovery biomarkers or predictors obtained by selected studies were compared in between case and control groups. A value of P<0.05 was interpreted as statistically significant. The I² index was used to assess heterogeneity between studies [13,14]. Random effect and fixed effect models were used to calculate the mean effect size of studies with significant heterogeneity (I² >75%) and without significant heterogeneity (I² <75%), respectively. For systematic review and risk of bias assessment, Review Manager 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014 was used [15].
Results
Study Selection
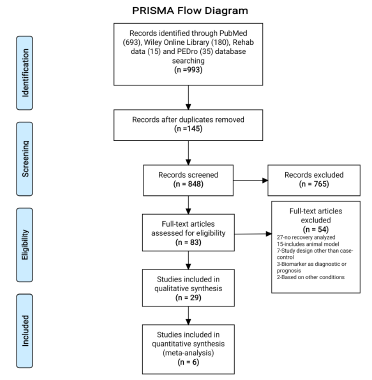
A total of 993 potentially relevant studies were identified using online databases (PubMed, Wiley online library, Rehab Data and PEDro Database) through a systematic search strategy. The 145 duplicates were found and removed after combining the studies obtained from different databases. After screening the titles of 848 studies, 765 studies were excluded, as they were found irrelevant. Further abstract screening of the remaining 83 studies found 32 studies ineligibles as study design was other than case-control, animal models, diagnostic or prognostic markers, based on other conditions and no recovery was assessed. Thus, a total of 51 studies were identified for full text screening. Identified full text studies were retrieved and screened for the eligibility. After detailed evaluation, 29 studies were found to be eligible for final analysis and measuring at least one or more of the considered outcomes in this systematic review [7,16-43]. The PRISMA flow diagram for selection of studies has been represented in (Figure 1).
Study Characteristics
The results of this systematic review are based on 29 studies involving 2528 participants. Out of 2528 participants, 926 were in the case group (patients diagnosed with ischemic stroke) and 1602 were in the control group (healthy volunteers). The average age and range of pooled patients were 59.49±5.98 years and 21-93 years, respectively. The results of 12 studies were based on acute stage stroke patients, nine studies on chronic stage, and three studies on sub-acute, two studies on combination of acute and chronic stage and in remaining three studies stage was not specified. Thus, the majority of participants (567/926) in the case group were found with acute stage of Ischemic stroke while, 237/926 participants were with chronic stage. The average months after the occurrence of stroke, when recovery was assessed in participants were found to be 19.36±27.55 months.
The majority of studies (20/29) reported motor recovery while few studies reported cognition recovery (3/29), sensory recovery (2/29), combination of motor and cognition recovery (3/29) and combination of motor and sensory recovery (1/29). Total 27 different measures were used to capture the outcome (recovery) in the participants enrolled in the included studies. The most common used measures of recovery were: General measures- National Institute of Health Stroke Scale (NIHSS), Modified Ranklin Scale (MRS); Motor recovery measures- Fugl Meyer Assessment (FMA), Wolf Motor Function Test (WMFT), Action Research Arm Test (ARAT), Arm Motor Ability Test (AMAT), Motor Assessment Scale, Finger Tapping (FT), Ashworth Scale, Chedoke-McMaster Stroke Assessment (CMSA), Barthel Index (BI), Grip strength, and Edinburg Handedness Inventory (EHI quotient); Cognition recovery measures-Mini–Mental State Examination (MMSE) and Montreal Cognitive Assessment (MCA). The publication, population, intervention and outcome characteristics of all selected studies are presented in the Appendix Table 1.
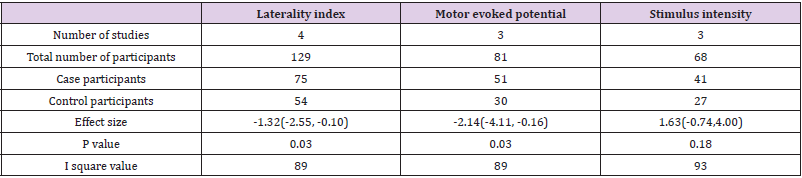
Table 1: Overall effect estimate of laterality index, motor evoked potential and stimulus intensity.
Biomarkers and Predictors
A total of 22 markers were reported in this systematic review. Further, these markers were categorized in sections: Motor Evoked Potential (MEP), Laterality, Cortico-Spinal Tract (CST), brain oscillatory activity, brain connectivity and activation, location, size and volume, cerebral blood flow and others. The details of the markers and their association with the recovery in Ischemic stroke patients are presented in the Appendix Table 2. Predictors like MEP (onset, amplitude, area and selectivity), laterality (laterality changes and index), brain oscillatory activity (event related desynchronization), brain connectivity and activation (resting state functional connectivity, ipsilesional cortico-cerebellar functional connectivity, brain activation and stimulus intensity), CST (damage, weighted CST lesion load) were found positively correlated with the recovery in ischemic stroke. On another hand, predictors like lesion size, infarct volume, lesion topography and white matter integrity were found negatively correlated with the recovery.
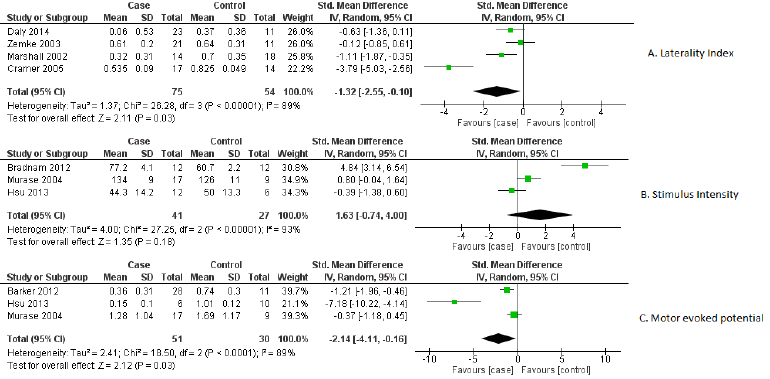
Out of 22 markers, three relevant markers were analysed by performing meta-analysis. The performance of these markers was measured in terms of their capability of differentiating the recovery of ischemic stroke from healthy participants. The standardized mean difference value of markers was used as an outcome measure for differentiation between case-control groups. As a significant heterogeneity was found, therefore the random-effects model was chosen over the fixed effects model. These relevant biomarkers differentiated ischemic stroke’s recovery from controls with good performance: MEP [standardized mean difference -2.14, 95% CI (-4.11, -0.16), P =0.03], laterality index [standardized mean difference -1.32, 95% CI (-2.55, -0.10.), P =0.03] and stimulus intensity [standardized mean difference 1.63, 95% CI (-0.74, 4.00), P =0.18]. The individual study effect sizes were reported in the forest plots based on outcomes. In view of the study wise reporting of markers, sample size was the highest for laterality index (129 participants), whereas the sample size for MEP and stimulus intensity were 81 and 68 participants respectively. The overall effect estimates of laterality, MEP and stimulus intensity were represented in (Table 1). The visual examination through the forest plot reflects that laterality index and MEP markers favours the case group. While the marker stimulus intensity favours the control group. The forest plot of these above mentioned three markers were represented in (Figure 2).
Figure 2: Forest plot of biomarkers
A) Laterality index,
B) Stimulus intensity and
C) Motor evoked potential.
Quality of Studies
Three parameters of quality: selection, comparability, and outcome/exposure were measured through NOS scale. One study was found to have high quality (nine point’s altogether), 23 studies had medium quality (seven-eight points) and five studies were poor quality reports (≤ six points).
Risk of Bias
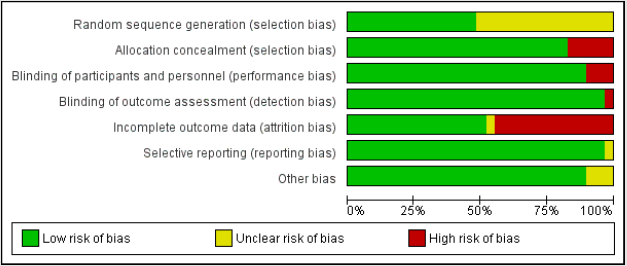
Mainly selection and attrition bias were observed within the selected studies. In few studies reporting and performance biases were also detected as the outcome was found to be reported only for selected groups. The risk of bias was considered adequate for the outcomes. The risk of bias for individual study and their summary were represented in (Figures 3 & 4) respectively.
Discussion
This review has systematically evaluated the biomarkers and predictors of recovery in ischemic stroke as well as their association with the recovery. A number of studies have assessed the association of various markers with the recovery after stroke. Among these, studies with case-control design are growing in number to determine the best predictor which can differentiate recovery in stroke patients from severely affected ones as well as healthy participants. To the best of knowledge, this is the first systematic review based on a case-control design to evaluate the markers which can predict and differentiate the recovery after the stroke. Through the comprehensive literature search, a total of 22 rapidly growing markers were found to be associated with the recovery after stroke. These markers were further categorized into eight broad categories in this review. The results of this review indicate that out of 22 markers, the laterality index and MEP were the only biomarkers that show relevant association with recovery. Hayward, et al. [44] reported MEP as the relevant biomarker to predict motor recovery which is consistent with our results. In addition, the results of this study demonstrated that the laterality index is also strongly associated with the outcome. Single- or repetitive-pulse stimulation of the brain causes the spinal cord and peripheral muscles to produce neuro electric signals known as MEPs [45]. MEPs or their absence serve as indications of the cortico motor pathway’s functional integrity and excitability, facilitating the assessment of associated motor impairment at the time of testing [46]. In this review, different variables of MEP such as MEP onset, MEP amplitude and MEP area were also found to be strongly associated with the recovery. The presence of MEP indicated better recovery and the mean values of MEP amplitude were found to be higher in healthy subjects than ischemic stroke participants.
Aside from MEP, the laterality index was revealed to be a relevant biomarker in this study. The laterality index is a method of determining hemisphere dominance in a range of activities, including language, cognitive skills, and changes in laterality in clinical populations, as in post-stroke condition [47]. In post-stroke fMRI investigations, it has been used to identify neuro plastic alterations in stroke survivors [37]. Further, it was found to be associated with greater activation in the hemisphere contralateral to the working limb (lesioned hemisphere) versus the hemisphere ipsilateral to the working limb (non-lesioned hemisphere) [24]. In this review, the laterality index was mainly extracted from functional MRI (fMRI) in most of the included studies and revealed that stroke is associated with a less lateralized pattern of activation when compared to healthy participants. It further indicated, more the shift towards the normal state of brain function is associated with a better recovery stage after stroke. In contrast to MEP and the laterality index, the pooled values of brain stimulus intensity were identified as statistically insignificant biomarker in this review. Increased stimulus intensity has been linked to a reduction in response time which represents rapid sensory and perceptual processing manifested in the presence of more intense physical stimuli. Further, in stroke survivors, the role of stimulus intensity was also detected in preparing voluntary movements using various techniques such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) [48]. However, the stimulus intensity for tDCS cannot be individualized based on motor thresholds as for repetitive TMS and may produce variable effects between individuals [39].
In this review the pattern of mean values in the case of brain stimulus intensity was found to be higher in ischemic stroke participants than healthy participants. The mean values of markers of the CST (CST integrity), brain oscillatory activity and connectivity were slightly higher in healthy subjects. The markers like lesion size, location, and infarct volume and lesion topography were not found to have any significant association with the recovery. Authors also want to address the limitations of presented study. Our literature search was constrained to articles only in the English language, which creates the possibility to have somewhat biased results. The outcome measurement in all the included studies was made with a variety of tools and different endpoints. Due to the limited individual patient data, pooling was not possible for all the biomarkers. Thus, it further suggests future studies to investigate recovery biomarkers mainly for cognition and sensory recoveries after stroke.
Conclusion
This review concludes that laterality index, MEP and stimulus intensity are the most relevant biomarkers to predict motor recovery in ischemic stroke patients. The biomarkers of cognition and sensory recoveries, association of markers with individual recovery as well as combination of recoveries (like sensory & motor, cognition & motor and sensory, cognition & motor) need to be investigated more in future studies to predict the recovery with greater precision.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.