Dynamics of P Under Redox Conditions in Rice in Tropical Soils
Introduction
Rice is one of the main foods in the world, due to its nutritional content and because of the ease of handling and adaptation in different parts of the world, especially in tropical and subtropical conditions [1]. On the other hand, this crop is established under different types of management, depending on the genetic material to be managed, the type of tillage, agronomic management, fertilization plans and irrigation management [1]. Among the types of risks to be managed in rice cultivation, there is the condition of constant sheet or flooding, which has the advantage of properly managing weeds, avoiding stress in areas with high temperatures, reducing specific phytosanitary problems in each region; however, soil fertility is affected by the high and low availability of some nutrients [2,3]. Among the elements that are affected by its nutritional dynamics is Phosphorus (P), an element determined as a macronutrient and which is required to fulfill specific functions of the plant related to the production of energy (ATP) [4], in addition to the importance it has for the production of tropical agricultural systems [5].
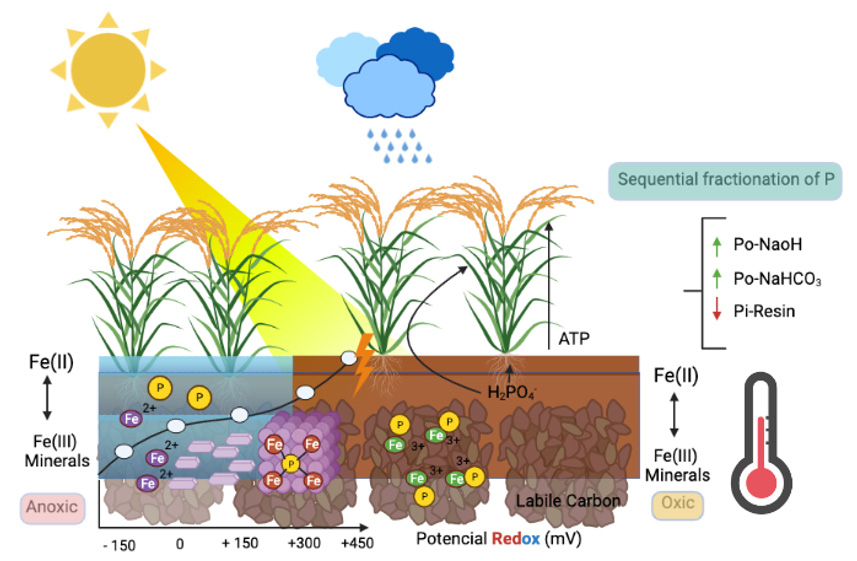
The behavior of this element is not the same when humidity is present at field capacity, as when flood irrigation is used in anoxic conditions, this, due to the fact that the concentrations of O2 in the soil are affected, the pH and pH are also affected. the dynamics of microorganisms that affect the available forms of P, as well as the concentration of elements such as Iron (Fe+2) that is found in the vast majority of tropical and subtropical soils in the form of minerals of high or low crystallinity. which directly influences the availability of P and the adsorption and desorption processes, in its Fe-P relationship, especially in acid soils where Fe is found in high concentrations and closely related to the oxidereduction conditions known as Redox conditions, which they are subject to the activity of electrons, pH and concentration of O2 in the soil; However, for this availability of P to exist, there are other parameters that must be present in the soil, such as high fertility defined by the cation exchange capacity (CEC), organic matter (OM) and especially dissolved organic carbon (DOC ) and a texture with high clay content [6,7], all this is described later as also in (Figure 1).
Figure 1: Dynamics of P in acidic soils in Redox processes in rice cultivation. Created with BioRender.com.
Relationship of pH and Eh in the availability of P
Is important to establish that flood periods do not generate immediate changes in pH, but can vary from a few to several weeks, among the factors that can establish changes in pH in flooded soils are: concentration of organic matter (dissolved organic carbon), Microbial activity, temperature, Fe concentration, ammonium accumulation, among other chemical properties of the soil [8,9]. Conditions such as pH and Eh are indirectly responsible for the release of P under reduced soil conditions [10,11]. After there is reduction in the soil by agronomic management such as flooding, regardless of the pH, which generally in tropical soils is very strongly acidic (<4.5) in soils of the orders Oxisol and ultisol, it has a tendency to rise until reaching the agronomic neutrality (6.5-6.8) [12,13], this is due to the fact that processes of reduction of MnO2 and Fe2O3 occur, to the production of OH- as a result of the mineralization of organic N to NH4 + through the process of the In the same way, the increase in pH can also be attributed to the rapid microbial mineralization of labile Carbon compounds (C), generating a rapid decarboxylation of organic anions and in addition to the ammonification as already mentioned above [14].
On the other hand, there is also a decrease in the redox potential (Eh), which is defined as a measure of the reduction state because it changes with the Oxidation / Reduction ratio; Eh is affected by the formation of complex ions and by pH [15]; It can be said that Eh decreases when the pH increases, therefore, the order of the compounds in which they are reduced in flood conditions in rice can be defined, which is described below: NO3 - = MnO2> Fe2O3 (Eh Low). In a soil with high Fe2O3 content such as oxisols, Eh remains an average of + 100 mV, however, in soils with little Fe2O3, Eh can range from -100 to -400 mV where the reduction of SO4 -2 to S-2 [16,17]. In the end, there is a correlation between pH and availability of P, studies carried out by [11] established that the increase in pH in acid soils (Oxisol and Ultisol), under flood conditions in rice, which indicated that these increases in pH, facilitated the Fe (III) reduction and associated P mobilization in the acid soils evaluated [18,19]
Dynamics of P and Fe Concentration
Lower Eh initial conditions in acidic soils such as oxisols [11], can result in a strong decrease in the sorption force of Fe-OP, leading to a strong increase in the P available in this type of soils [20,21]. In relation to this, it can be said that a reduction of Fe is more likely to occur at a slightly acidic pH, when the increase in pH is occurring in reducing environments [22]. It is important to bear in mind that potentially reducible Fe can be established, through methodologies such as the determination of Fe with Citrate-Ascorbate (FeCA), which indicates that the total concentrations of Fe (III) and recalcitrant Fe (Easily reducible) control the production. Fe (II) in acid soils such as Oxisols and Ultisols [11,23]. It is important to note that Fe (II) has a lower binding force for P than Fe (III) [24]; therefore soils with higher potentials to reduce Fe, an early increase and a higher bioavailability of P can be expected [22]. The reducing dissolution of crystalline Fe (FeCA, defines Fe and low crystallinity and potentially available and reducible Fe) can be catalyzed by a high concentration of Fe (II) [25,26], resulting in a higher initial availability of P in acid soils [11,27]. In short, the increase in the availability of P in flood conditions has to do with the reduction of ferric phosphates (Fe+3) to ferrous phosphates (Fe+2), to the release of P from insoluble components of Fe and Al and to a certain dissolution of Ca phosphates when there are high levels of CO2 in the soil solution (soils with alkaline pH). The release of P through these processes can take a few weeks after the flood. This initial flux of released P can be fixed on clay particles and Al hydroxides (AlOOH), and in some soils with high amounts of active Fe and Al it can still result in a reduction in the availability of P in the soil [28,29].
Organic Matter and P Concentration
Labile organic acids are more efficient to solubilize P from acidic soils, this because there is a greater solubility of Fe and Aluminum (Al) phosphates, with increasing pH and low Eh (Reference). Therefore, a decrease in Eh, followed by a reduction in Fe (III), can generate the release of P bound in Fe minerals; The reduction of Fe (III) and the corresponding accumulation of Fe (II), strongly depend on the labile mineralization of C and OM. Labile C can define the intensity of Fe (III) reduction because it is the main source of energy for microorganisms, in addition to being a strong electron donor [30]. These processes result in the production of Fe (II) but in the same way a desorption producing available P; therefore, higher OC and Fe (II) content in soils such as oxisols, generates a drastic decrease in Eh after immersion [11], which leads to a greater consumption of microbial O2 much faster, which can generate the release of large concentrations of P low binding energy (Reference). It is important to bear in mind that microbial mineralization with rapid activities due to the presence of labile C in acid soils can reduce P sorption, a specific adsorption analogous to phosphate ions [31] or by Fe and Al chelation [32].
Conclusion
It is important for the researcher, extension worker, professor in the area of agriculture, to understand the dynamics of P in systems where Redox processes are present, since for the producer it will serve as an orientation not to carry out high fertilization of phosphate fertilizers, which can lead to high processes of contamination by eutrophication, cost overruns, currently necessary due to the costs of fertilizers that have doubled and tripled their price in the market, in addition to the need to carry out calibrations in the determination of P in the soil, since in the tropics There is determination of P by the method of Bray II (Colombia), Mehlich 3 (Brazil - Minas Gerais), Resina (Brazil - São Paulo and Rio Grande do Sul), generating ambiguity by establishing non-standardized methodologies in various regions of the tropics and by Finally, in addition to values such as pH in the soil analysis, one should work on the fractionation of P as results in the soil analyzes for all The agricultural professional (Agronomist Engineer, Agroecologist Engineer, Agroforestry Engineer, etc.) who is the person in charge of the recommendation, with this, the forms of P, labile, moderately labile and nonlabile are determined to make a much clearer and more accurate recommendation.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.