Omicron (B.1.1.529): Variant of Concern – A Mini Review
Introduction
Different variants of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) have been identified since the first coronavirus disease 2019 (COVID‐19) infection appeared in December 2019. Until November 2021, the Delta variant was designated as variant of concern (VOC) because of different characteristics [1]. According to the Centers for Disease Control and Prevention (CDC), the variant responsible for increased transmissibility, severe disease course, reduced effectiveness of treatments, and many other alarming factors is designated as the VOC [2]. On 26 November 2021 the World Health Organization (WHO) Technical Advisory Group on SARS-CoV-2 Virus Evolution named the B.1.1.529 COVID-19 variant, first detected in Botswana and South Africa, as the Omicron variant of concern [3]. This variant carries an unusually high number of mutations, 32, on the spike (S) protein, the main antigenic target of antibodies generated by either infections or vaccination. The B.1.1.529 COVID-19 classification was based on a rapid increase in cases in South Africa, coinciding with an increase in detections of Omicron, a number of concerning mutations and early evidence suggesting an increased risk of reinfections.
The overall threat posed by Omicron largely depends on four key questions:
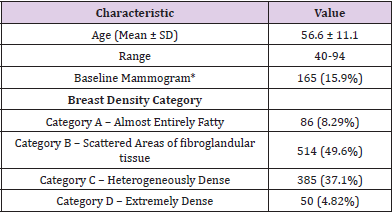
i. How transmissible the variant is;
ii. How well vaccines and prior infection protect against infection, transmission, clinical disease and death;
iii. How virulent the variant is compared to other variants; and
iv. How populations understand these dynamics, perceive risk and follow control measures, including public health and social measures (PHSM) [4].
Based on the currently available evidence, the overall risk related to Omicron remains very high. Omicron has a significant growth advantage over Delta, leading to rapid spread in the community with higher levels of incidence than previously seen in this pandemic. Despite a lower risk of severe disease and death following infection than previous SARS-CoV-2 variants, the very high levels of transmission nevertheless have resulted in significant increases in hospitalization, continue to pose overwhelming demands on health care systems in most countries, and may lead to significant morbidity, particularly in vulnerable populations [4]. The identification of Omicron as a variant of concern (VOC) by the World Health Organization (WHO) has triggered countries around the world to put in place travel restrictions and precautionary measures. Therefore this work aims to review Omicron variant impacts, transmissibility, viral infectivity, genomics and efficacy of existing vaccines.
Genomics of Omicron Variant
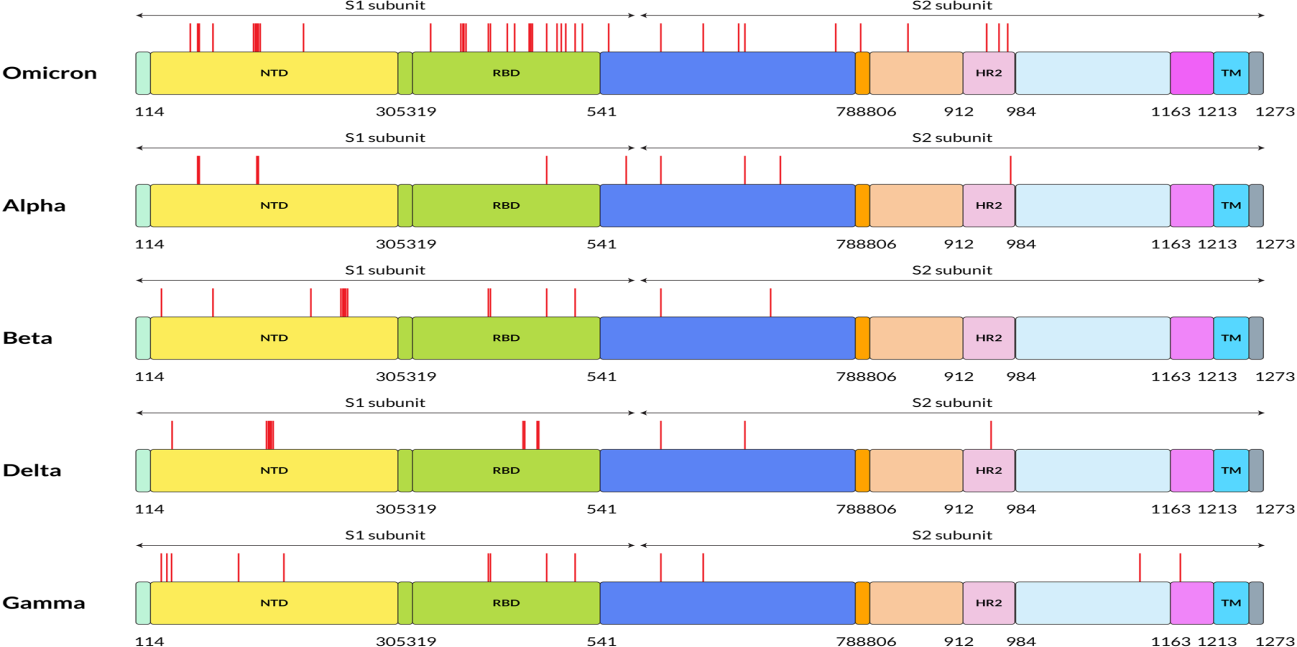
The unique genome features of the Omicron variant make it the most special SARS-CoV-2 variant to date. The genome constitutes 18261 mutations from which more than 97% mutations are present in the coding region, and the remaining 558 are detected in the extragenic region [5]. Mutations in the coding region are 2965 indels and non-synonymous, and synonymous singlenucleotide polymorphisms (SNPs) mutations are 11 995 and 2743, respectively [5]. Thirty mutations have been found within the spike proteins mostly located at the receptor‐binding domain (RBD) of the spike protein of the Omicron variant [6]. The excess number of nonsynonymous mutations in the Spike gene implies that the Omicron variant might evolve under selection pressure, which may come from antibodies or adaptation to new hosts. Reports show that there are three other deletions and one insertion mutation outside the spike protein (Figure 1). Preliminary data analyzed from the Global Initiative on Sharing All Influenza 101 Data (GISAID) showed that NTD contains 11 mutations, including six deletions and one insertion, with mutations N211 and ins214EPE being unique [7]. Interestingly, some of the mutations were already found in the previous VOC that helps in neutralizing antibodies [8,9]. Five different variants of SARS‐CoV‐2 have been considered VOC at different times [10]. Investigations performed by epidemiologists in South Africa identified the mutational data that manifests some of the concerning mutations (N501Y, D614G, K417N, and T478K) along with new mutations present in the Omicron variant, which increased the overall risk of reinfection, partial resistance to existing vaccines [10].
Figure 1: Five SARS-CoV-2 variants have different amino acid mutation locations on their S proteins. On this figure, red lines indicate the mutation locations on the S protein of SARS-CoV-2 at the specified positions. FP stands for fusion peptide, and pink color specifies HR2: heptapeptide repeat sequence 2, TM: transmembrane domain, which showed in sky shade, NTD: N-terminal domain shown in yellow and green color denotes RBD: receptor-binding domain.
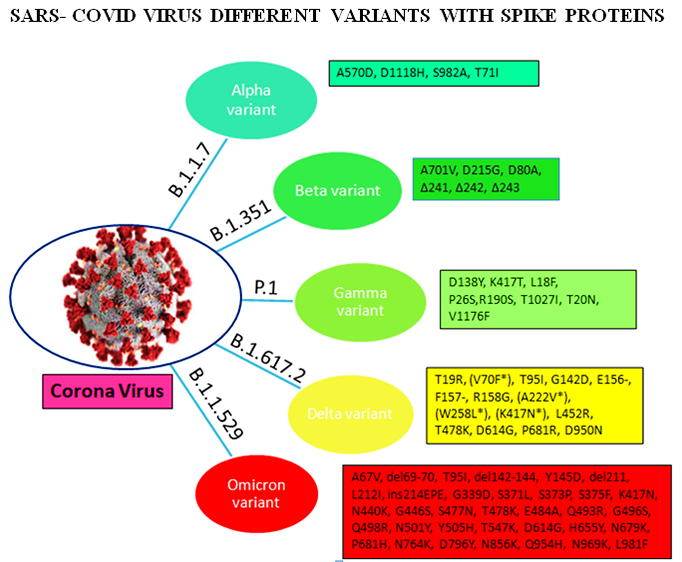
Omicron variant is known to boost affinity for ACE2 receptors found on the surface of various human cells, including the lungs [11]. Even though Wuhan‐Hu‐1 has 1273 amino acids, the Delta variation has 1271, and the Omicron variant has 1270, both contain fewer residues than the wild‐type due to sequence loss [12]. Genome analysis by Kandeel et al. reported that the Omicron variant of SARS‐CoV‐2 forms a new monophyletic clade [13]. On the other hand, Wang et al. showed that the Omicron variant of SARSCoV‐ 2 evolved from the 20B clade and formed two sub-clades [14]. On the other hand, many of the variants were observed with the different protein sequences of the genome. The alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2), and omicron (B.1.1.529) variants are among the variants. These variants have different protein sequences as shown in Figure 2.
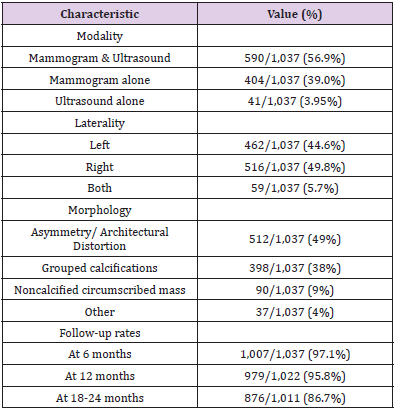
Transmissibility and Infectivity of Omicron variant
There is still a scarcity of sufficient essential data regarding the infection rate to analyze the transmissibility of the new heavily mutated Omicron variant. However, analysis from the early data of South Africa manifested that the Omicron variant can spread way more easily from person to person, though experts could not draw any conclusion within this short period [15]. The concern of Omicron variant transmissibility increases as it spreads worldwide within a few days, and cases have been increasing dramatically [16]. According to the report of CDC, a 2.5% increasing capacity of Omicron variant has been observed in the US within 2 weeks. However, in New York/New Jersey area, the infection rate is around 13%. On the other hand, in Britain, Omicron variant cases doubled every 2–3 days [15]. The infection rate of the Omicron variant in South Africa is increasing faster than any other country’s three previous waves. On November 30, the number of cases was 10.3%, shifting to 16.5% within two days. Surprisingly, on December 2 and 3, cases were 22.4% and 24.3%, respectively [17]. When the linear regressions of each pseudo virus were compared to the wild type over the entire range, it was discovered that while the Gamma variant had similar infection rates to the wild type, the Beta variant had less infection, and Delta was nearly twofold more efficient at infecting target cells. Infection rates were four times higher in the Omicron variant than in the wild type and twice as high in the Delta variant. These findings indicate that spike sequence influences infectivity, with the Omicron variant displaying more effective ACE2‐mediated infection than the wild type or other variant strains [18].
The infectivity of SARS-CoV-2 is mainly determined by the binding affinity of the ACE2 and RBD complex, although the furin cleavage site plays a crucial role as well [11]. Omicron has three mutations at the furin cleavage site and 15 mutations on the RBD, suggesting a significant change in its infectivity. Due to natural selection, the virus enhances its evolutionary advantages at the RBD either by mutations to strengthen the ACE2-RBD binding affinity or by mutations to escape antibody protection [12]. Since the virus has optimized its infectivity in human cells, one should not expect a dramatic increase in the viral infectivity by any single mutation. An effective infection pathway is for the virus to have multiple RBD mutations to accumulatively enhance its infectivity, which appears to be the case for Omicron. Furthermore, the new variant Omicron gives a false negative result in polymerase chain reaction tests because of the “S gene target failure,” which paves the way of spreading the infection at a higher speed worldwide [19]. Numerous factors can influence the high transmissibility of the Omicron variant. Genome sequenced data of the Omicron variant demonstrated more than 30 mutations in the spike protein by which the SARS‐CoV‐2 protein recognizes host cells [20]. Analysis of these mutations data indicates the chance of increased transmission by evading the immune response [21]. The N501Y mutation increases the binding affinity with the ACE2 receptor, which is a major influencer of increased transmission, and in combination with Q498R, the binding affinity gets stronger, and the Omicron variant gets easy access into the host [21]. Moreover, the risk of reinfection of previously COVID‐19 infected patients with the Omicron variant is very evident, indicating higher transmissibility [8]. Omicron variant mutations H655Y and N679K are present near the furin cleavage site (FCS) and can increase spike cleavage, making the virus more contagious [22,23]. On the other hand, P681H can multiply transmissibility by increasing the spike protein cleavage [24].
Disease Severity
Globally, there has been a 4% increase in the number of new deaths from in week 2 (10 – 16 January 2022) compared to the previous week, with highest increases in the South-East Asia Region (12%) and the Region of the Americas (a 7% increase). Data on case severity (including hospitalization, need for oxygen, mechanical ventilation, or deaths) are increasingly becoming available, improving our understanding of the impact of Omicron on severe cases, hospitalization and deaths. Surveillance trends from most countries show a decoupling between incident cases and hospitalizations in many countries, with proportionally lower incidence of hospitalization, given the level of community transmission, than what was observed with other variants. This decoupling appears to be driven partially by a lower intrinsic severity of Omicron compared to Delta, as well as by more preserved vaccine effectiveness against severe disease than against infection. Several studies have looked at the risk of hospitalization and severe disease with Omicron compared to Delta. The most recent analysis from the United Kingdom Health Security Agency with the Medical Research Council (MRC) Biostatistics Unit, University of Cambridge showed a 47% reduction in the risk of presentation to emergency care or hospital admission with Omicron compared to Delta and 66% reduction in the risk of admission from emergency departments [25]. A report by Imperial College London on 22 December 2021 [26] calculated a 41% reduced risk of a hospitalization resulting in a stay of one or more nights. Similarly, using a record linkage approach [27], a study in South Africa found that laboratoryconfirmed SARS-CoV-2 infected individuals with SGTF, as a proxy for Omicron, had lower odds of severe disease.
COVID-19 Vaccine Efficacy on Omicron Variant
The Omicron variant of SARS‐CoV‐2 was identified from the COVID‐19 vaccinated patients, suggesting the new variant’s immune invasion and demanded updated vaccines [28]. Saxena et al. analyzed the mutations reported in the RBD of the spike of Omicron variant of SARS‐CoV‐2 and hypothesized that currently, available entry inhibitors might not be effective for emerging variants [29]. The heavy mutation in the spike protein of the Omicron variant is related to increased infectivity and antibody evasion [30]. In SARSCoV‐ 2 convalescent or vaccinated people, the amount of neutralizing epitopes targeted by polyclonal antibodies is a significant predictor of the genetic barrier to viral escape. Single monoclonal antibodies are susceptible to escape mutations, but combinations targeting non-overlapping epitopes are more resistant [31]. Surprisingly, Omicron variant neutralization was undetectable in the majority of vaccines [7]. The computational approach also demonstrated that antigenic properties of the Omicron variant are ominous and correlated with its mutations [32]. Although various investigations have been performed to create effective vaccines, the emergence of new VOCs has raised concern over the efficacy of neutralizing antibodies induced by COVID‐19 vaccines as the Omicron variant has already infected vaccinated individuals in South Africa, Hong Kong, and many other countries [30,33,34].
The potential impact of the COVID‐19 vaccine is still being analyzed against this new variant. Two BNT vaccinations, which can provide more than 90% protection against serious disease when infected with the Delta variant, maybe significantly less effective against the Omicron type of SARS‐CoV‐2 [7]. However, the effect of COVID‐19 vaccines against the previous VOC, such as Delta, manifested the vaccine’s potential in reducing severe disease and death [35]. Moreover, multiple Delta transmissions from and between completely vaccinated persons were confirmed using genomic and epidemiological data [36]. As vaccine‐induced immunity is targeted through the spike proteins of the virus, heavily mutated Omicron variant spike protein is capable of reducing the neutralization activity of sera of vaccinated individuals that indicated less protection from Omicron variant [21].
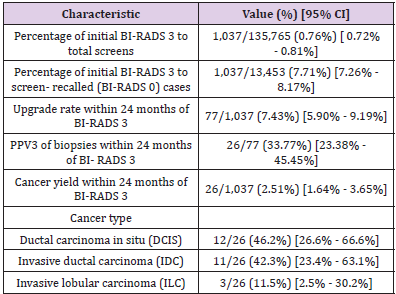
Various Impacts of Omicron Variant
The current evidence on the potential impact of the Omicron variant reported by World Health Organization is explained briefly [4].
Impact on Epidemiology: As of 20 January 2022, the Omicron variant had been identified in 171 countries across all six WHO Regions. Omicron has a substantial growth advantage over Delta, and it is rapidly replacing Delta globally. There is now significant evidence that immune evasion contributes to the rapid spread of Omicron. Data on clinical severity of patients infected with Omicron are increasingly available. Epidemiological trends continue to show a decoupling between incident cases, hospital admissions and deaths, compared to epidemic waves due to previous variants. This is likely due to a combination of the lower intrinsic severity of Omicron, as suggested by a number of studies from different settings, and that vaccine effectiveness is more preserved against severe disease than against infection. However, high levels of hospital and ICU admission are nevertheless being reported in most countries, given that levels of transmission are higher than ever seen before during the pandemic. This is particularly important given that current evidence about severity and hospitalization has largely been shared from countries with high levels of population immunity, and there remains uncertainty about the severity of Omicron in populations with both lower vaccination coverage and lower prior exposure to other variants.
Impact on Diagnostics and Testing: The diagnostic accuracy of routinely used PCR and the WHO emergency use listing (EUL) approved antigen-detection rapid diagnostic tests (Ag-RDT) assays does not appear to be significantly impacted by Omicron. Most Omicron variant sequences reported include a deletion in the S gene, which can cause an S gene target failure (SGTF) in some PCR assays. As a growing minority of publicly shared sequences (including all BA.2 sub-lineage sequences) lack this deletion, using SGTF as proxy marker to screen for Omicron will miss Omicron lineages lacking this deletion.
Impact on Immunity: Current evidence consistently shows a reduction in neutralizing titres against Omicron in individuals who have received a primary vaccination series or in those who have had prior SARS-CoV-2 infection. In addition, increased risk of reinfection has been reported by South Africa, the United Kingdom, Denmark, and Israel. There is a growing body of evidence on vaccine effectiveness (VE) for Omicron, with data available from 15 observational studies from five countries (the United Kingdom, Denmark, Canada, South Africa, and the United States of America), evaluating four vaccines (mRNA vaccines, Ad26.COV2.S, and AstraZeneca-Vaxzevria). Available preliminary data should be interpreted with caution because the designs may be subject to selection bias and the results are based on relatively small numbers. Early data suggest that the effectiveness of studied vaccines is significantly lower against Omicron infection and symptomatic disease compared to Delta, with homologous and heterologous booster doses increasing vaccine effectiveness. Despite this, follow-up time after booster doses for most studies is short, and there is evidence of waning of VE in months following booster doses. VE estimates against severe outcomes, usually defined as hospitalization, are lower for Omicron than Delta, but mostly remain greater than 50% after the primary series and improve with a booster dose to above 80%. There are no effectiveness data for several vaccines, particularly the inactivated vaccines.
Impact on Host Tropism, Virus Fitness and Pathogenicity: Preliminary evidence suggests a potential shift in tropism of the Omicron variant towards the upper respiratory tract, as compared to Delta and the wild type (WT) virus that have a tropism for the lower respiratory tract. There is also evidence of less severe pathogenicity in the Syrian hamster (M. auratus) model, but this needs to be confirmed by peer-reviewed evidence and larger studies.
Impact on Therapeutics and Treatments: Therapeutic interventions for the management of patients with severe or critical Omicron-associated COVID-19 that target host responses (such as corticosteroids, and interleukin-6 receptor blockers) are expected to remain effective. However, preliminary data from nonpeer reviewed publications suggest that some of the monoclonal antibodies developed against SARS-CoV-2 may have impaired neutralization against Omicron. Monoclonal antibodies will need to be tested individually for their antigen binding and virus neutralization, and these studies should be prioritized. Preliminary in vitro data suggests that antivirals retain activity against Omicron.
Conclusion
Omicron (B.1.1.529) which has been designated as variant of concern (VOC) by World Health Organization (WHO) is distinct from the previous variants of SARS-CoV-2 (Alpha, Beta, Gamma, and Delta). Hence, the scientific community is searching for the impact of this new variant on the SARS-CoV-2, such as transmissibility, disease severity, diagnostic method, efficiency of existing vaccines and other COVID-19 protocols. This mini review has provided the latest data on the genomics, transmissibility and infectivity, diseases severity, vaccine efficacy and the current evidences on the variant impacts on epidemiology, diagnostics and testing, immunity, host tropism, virus fitness and pathogenicity and therapeutics and treatment. Meanwhile, the previous recommendations to tackle the COVID‐19 pandemic need to be maintained worldwide along with the newly improvised directions, such as genome sequencing of all the samples, maintaining social distance, continuing vaccination for everyone, and isolating the Omicron variant positive patients in a different place. WHO recommended countries strengthen surveillance and adopt necessary actions since the Omicron variant has been designated as a VOC.
For more Articles on: https://biomedres01.blogspot.com/