Design and Simulation of a Neutron Generating Target Based on the 9Be (P, N) 9B Reaction for Use in BNCT
Introduction
One way to kill cancer cells is to treat the absorption of boron neutrons. It is based on the reaction of 10B (n, α) 7Li, which releases two heavy charged particles that deposit their energy at a very short distance. A tumor search compound containing the stable isotope 10B enters the bloodstream and accumulates in the tumor. The tumor is then irradiated with epithermal neutrons, which are heated before being taken by isotope 10B. A typical definition is used for the epithermal energy range, i.e. 0.5 eV to 10 keV [1]. Damage due to dose delivery to tissue leads to cell death. Because the range of alpha particles and lithium nuclei is very short, healthy tissue around the damaged cell is safe [2]. The neutron source is essential for the success of BNCT. The energy and intensity of the source is of great importance. In most centers that use this method for treatment, research reactors are the source of neutrons. In practice, a large amount of unwanted radiation is produced for a deep tumor along the desired slow neutrons. Nuclear gamma rays receive gamma rays from the surrounding material, as well as backward proton reactions and 14N (n, p) 14C, which produce undesirable radiation. Hence, the accelerator-based neutron source (ABNS) has been favored in many applications, including BNCT [3,4].
Accelerators have advantages over research reactors such as higher safety, easier operation, less gamma ray emission, larger size, more cost-effective, easier start-up and shutdown, and the neutron energy spectrum can be adjusted for better treatment. In addition, ABNS can be placed near the hospital. Therefore, the development of the AB-BNCT system is a prerequisite for BNCT due to the increase in the number of volunteers. Several reactions can be performed in ABNS systems to produce neutrons such as 7Li (p, n) 7Be, 9Be (d, n) 10B, 9Be (p, n) 9B, 181Ta (p, n) 184W (p, n) used) etc. [5-7]. Today, the use of cyclotrons to generate neutron beams is a desirable method for the application of AB-BNCT. The combination of several tens of MeV protons and a desired beryllium produces a beam. A combination of several dozen MeV protons and a beryllium to generate a 1mA 30MeV proton beam is under consideration in this project. However, we confirmed that sufficient epithermalneutron efficiency based on the 9Be (p, n) 9B reaction can be achieved with an optimal beam-forming set. The advantage of this system is less activity and higher neutron efficiency compared to scaling reactions involving heavy materials. The Monte Carlo MCNPX 2.6 code is used to simulate the system to optimize the entire configuration and maximize the neutron flux. Neutron flux (beam intensity) determines the irradiation time. The flux must be strong enough for a reasonable irradiation time. A cooling system is required to increase the flux and reduce the irradiation time. We offer gallium cooling system for this purpose
Material and Methods
Neutron Beam
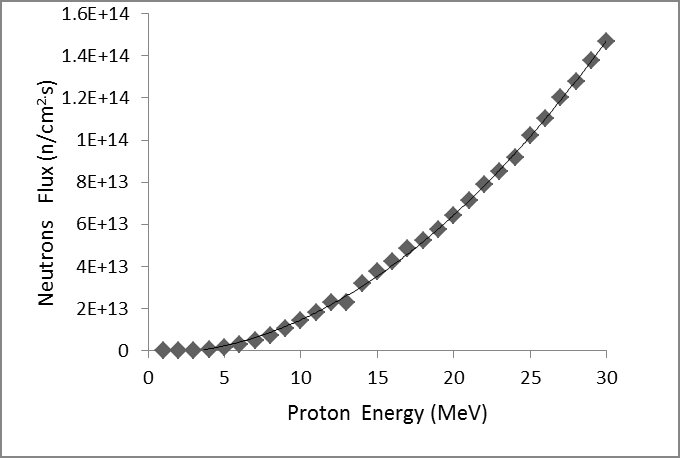
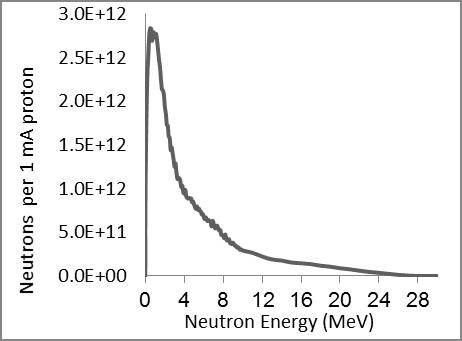
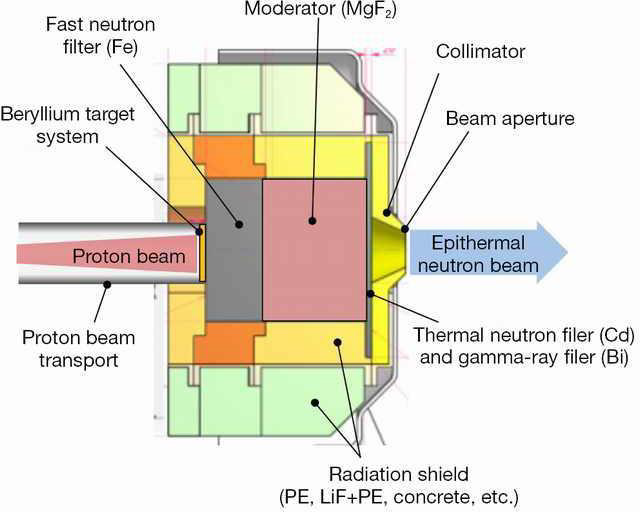
A system using the 7Li (p, n) 7Be reaction requires an accelerator with a current of> 5 mA to generate a 1x109 n/cm2.s epithermal neutron flux intensity (suitable for BNCT) and obtain It is difficult to bring. Heat production in lithium is the melting point of lithium is 180 degrees Celsius, which is much lower than beryllium (1278 degrees Celsius) [8,9]. In order to prevent additional radiation to the patient, the additional costs for remote control devices and activation of the heavy-duty shielding target should be minimized. Considering the maintenance, storage, and control objectives of the low activation is important for hospital management. Given these facts, we chose the 9Be (p, n) 9B reaction with 30 MeV protons, which has higher neutron efficiencies compared to the 7Li (p, n) 7B reaction, as well as lower activation levels than W and Ta [10,11]. Only one of the beryllium (9Be) isotopes is stable and is a primary nucleus. In this work, we aim to design a target system by simulation using MCNPX 2.6 code. We plan to use a cyclotron accelerator that promises a 1 mA proton beam at 30 mV, which generates maximum energy from our cyclotron at the Cyclotron Atomic Energy Research Institute. As shown in Figure 1, the neutron efficiency changes in reaction (p, n) with the proton energy.
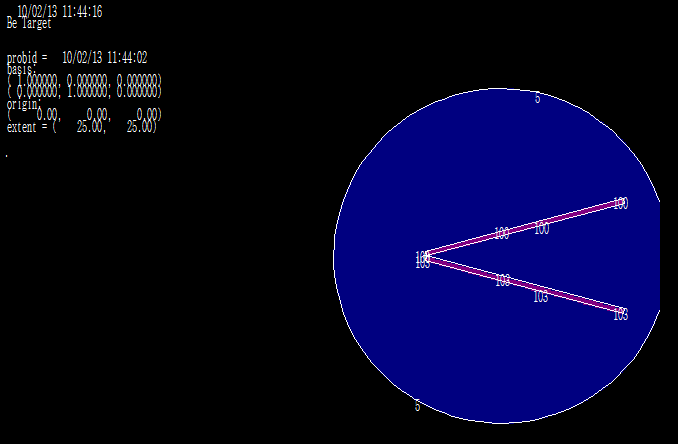
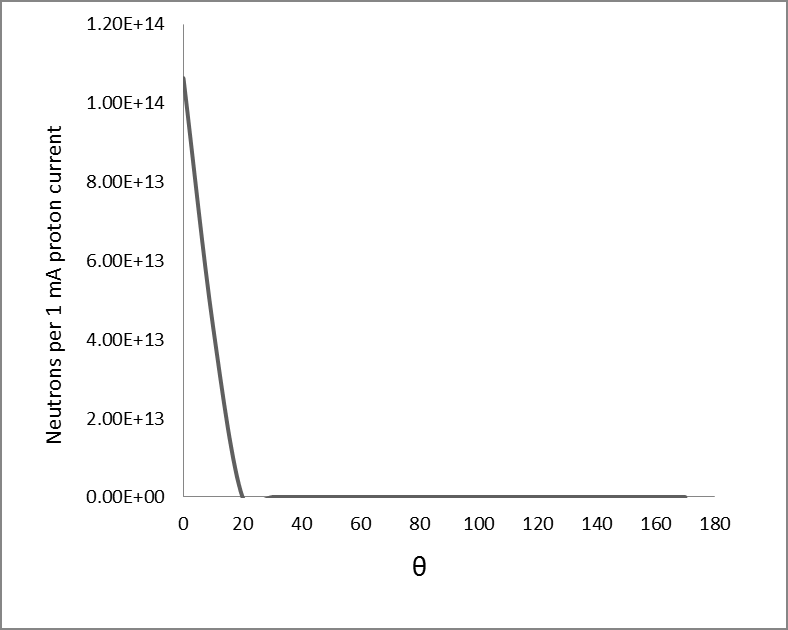
We used a uniform proton beam with a diameter of 8 cm as a simulation. We tried to find the best geometry for the highest neutron flux. So we decided to choose a V-shaped target, Figure 2 with an opening angle of 30 degrees, so that the whole proton beam joins most of the surface, and to get a compressed neutron beam, the heat generated at the same time is faster. Removed from the target level. Opening angle to various positions including; cooling system pump and proton beam. Beryllium is 5.5 mm thick, which is slightly shorter than the proton range of 30 MeV in beryllium (5.8 mm). Figure 3 shows the best simulated target for production. Simulations using MCNPX 2.6 showed that the neutron flux system showed 1.5 * 1014 (n / cm2.s) in a 1 mA proton beam, with an energy spectrum extending to 1 keV-28 MeV, as shown in Figure 3 given. Between the target beryllium and the surface detector. (Figure 3) Neutron flux 1.5*1013 (n /cm2.s) at 1mA proton beam, with an energy spectrum extending at 1keV-28 MeV. As shown in Figure 3, due to the use of high-energy protons, we have 28 MeV neutrons in this spectrum, so we can use a suitable beam-forming set (BSA) suitable for rapid neutron removal. The angular distribution of neutron efficiencies, derived from the code MCNPX2.6, is shown in Figure 4. As a result of the high current level, the target heats up quite quickly. To prevent heat from concentrating in the target, the proton beam can be rotated using a magnet. A 1 mA, 30 mV proton beam has a power of 30 kW.
The neutron efficiency distribution determined using the MCNPX 2.6 code is shown in Figure 5. A target cooling system is required, although we used a rotating beam. Heated surface temperature is a critical parameter for ABNS target design. Assuming 30 kW of power in an area with a diameter of 8 cm, the average heat flux is 600 watts per square centimeter. We choose gallium coolant to remove heat from the target surface. [12], as shown in Figure 6. Compared to other liquid metal coolers, gallium has advantages and disadvantages for this purpose. Low vapor pressure and high boiling point cause liquid gallium to remain in one phase even at high temperatures [13]. Its activation products have a short half-life. And most importantly, gallium is liquid at temperatures above 30 ° C.
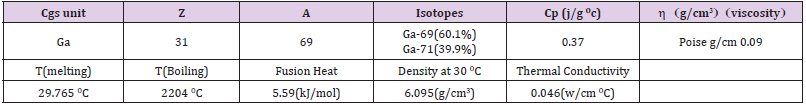
Properties of Gallium Are as Follow
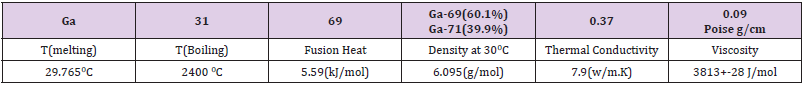
• Atomic number: Z = 31; Mass number: A = 69; Stable isotopes: Ga69 (60.1%), Ga71 (39.9%).
• Melting point: Tm = 29.82 °C; Welding temperature: Tb = 2403 ° C.
• Fusion temperature: Qf = 5.59 kJ / mol; Density at 30 °C: ρ = 6.095 g / cm3.
• Thermal conductivity: k = 0.406 W / cm °C. Specific heat: Cp = 0.37 J / (g. ° C).
•Viscosity: η = 0.019 Poison = 0.019 g / cm (3rd1000.com/elements/Gallium).
The range of 30 MeV protons in gallium is 2.5 mm, and if the protons stop in gallium cooling, they are not thought to cause a problem.
Calculations
The gallium flows through stainless steel channel. We assume that the flow channel is of rectangular cross section 80 mm wide and 3.5mm thick, so that the equivalent diameter is
De = 4 Ac / Pw = 0.76 cm [14].
The Prandtl number is: Pr = Cp.η / k = 0.0173;
The Reynolds number is: Re=De.v.ρ/η=2.15×104.
The Peclet number is: Pe = Pr.Re =371.95.
The Nusselt number is: Nu = 5.8 + 0.02(Pe)0.8 =8.0772
The heated diameter is: Dh = 4 Ac / Ph =0.35 cm;
And the heat transfer coefficient is: h = k.Nu / Dh =9.37 W/cm2.oC
Then the wall-fluid temperature difference is:
ΔT = q´´ / h= 64.034oC
h=k.Nu/Dh=0.406*8.0772/0.35=9.37W/cm2/oC
ΔT= q”/600h/ 9.37=64.0341oC
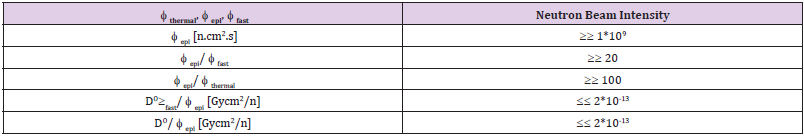
Table 1 lists several specific features. The critical physical properties of gallium isotopes at different temperatures are given in Table 1. At room temperature, gallium is softer than lead and can be cut with a knife. Its melting point is abnormally low and begins to melt in the palm of your hand. Gallium is one of the few metals that expands during freezing, Table 1. Poise is a cgs unit of liquid rendering viscosity to one dyne-second per square centimeter, = 0.019 Poise = 0.019 g / cm. Table 2 lists the critical properties of Li, Be, Ta and W. Some basic specifications such as temperature conductivity are given in Table 2.
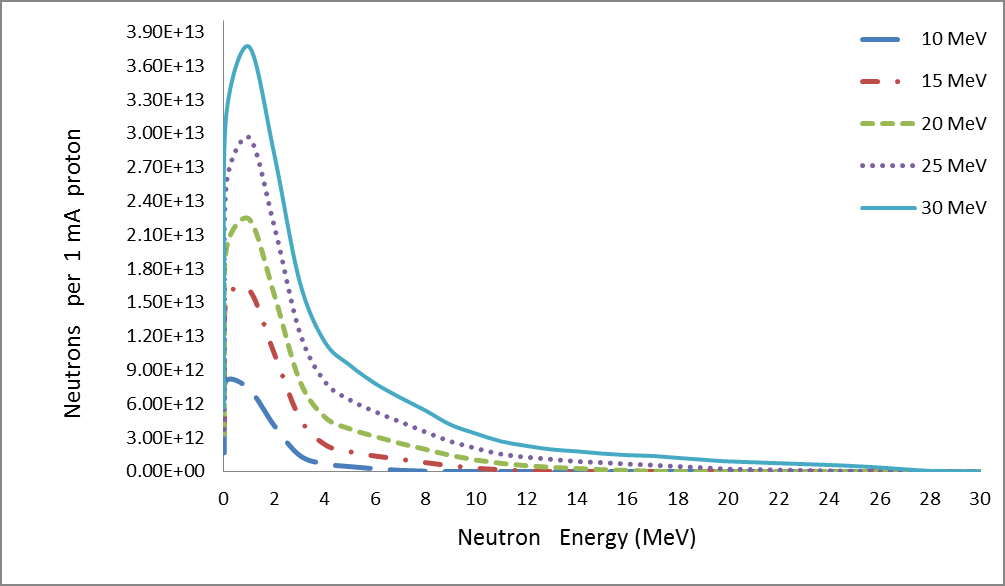
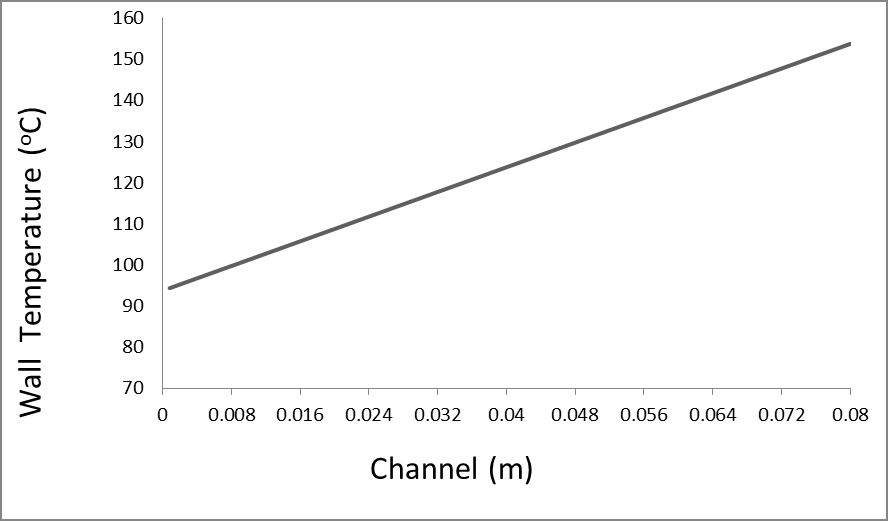
This figure is acceptable for the gallium cooling system. This structure prevents the proton beam from blistering the target and also reduces the target heat without reducing the neutron flux, Table 3. The result of our simulation using the MCNPX 2.6 code showed that the neutron flux system produces 1014 n/cm2.s with neutron energy spectrum extending from 1keV to 28MeV, with a peak of 0.8 MeV extending from 0.10 MeV to 3MeV, Figures 5 & 7. We used liquid gallium to cool the target in our design. This gallium cooling system maintains the target temperature 120 ° C above ambient temperature at average cooling rates (1 m / s). Irradiation time allows the irradiation to be completed while maintaining a high 10B concentration in the tumor in the BNCT and reducing the nonselective background dose. In addition, shortening the irradiation time makes the patient more comfortable during irradiation.
Result and Discussions
The result of our simulation by MCNPX 2.6 showed that this system produces a neutron flux of 1014n / cm2.s with a neutron energy range from 1keV to 28MeV, with a peak at 0.8 MeV that extends from 0.1 MeV to 3 MeV. We used liquid gallium to cool the target in our design. This gallium cooling system maintains the desired temperature 120 ° C above the ambient temperature with an average cooling rate (1 ml s). Therefore, by using this cooling system, we increase the efficiency of the target and reduce the treatment time. Shortening the irradiation time allows the irradiation to be completed while maintaining a high 10B concentration in the tumor in the BNCT and reducing the non-selective background dose. In addition, shortening the irradiation time provides patient comfort during irradiation. General requirements for BNCT reactor neutron sources, techniques for generating epithermal neutron beams starting from fission neutrons, and performance evaluation parameters will be briefly discussed. Design and simulation of a neutron generating target based on the 9Be (p, n) 9B reaction used in the BNCT process. The energy of effective neutrons is about 10 eV-4kV. Less energy neutrons are absorbed into healthy tissues before they reach the gland. Neutrons with energies higher than 10 keV due to proton excretion significantly increase the absorbed dose in healthy tissues (Supplementary Text).
Conclusion
In this work, we studied the use of a cyclotron as a neutron source for BNCT. For this purpose, a system called a neutron source based on a cyclotron has been designed. The target is simulated by MCNPX 2.6 to optimize geometry and dimensions. The designed system produces a sufficient neutron flux. An accelerator-based neutron source can be located near a BNCT center in a hospital. However, there are many things to develop and optimize about accelerators, modulators including targets, collimators, shields and so on. This could lead to the future development of BNCT therapy. In this paper, we report a brief review of fission reactor-based neutron sources for BNCT, focusing on the basic requirements that these sources are capable of meeting BNCT applications. General requirements for BNCT reactor neutron sources, techniques for generating epithermal neutron beams starting with fission neutrons, and performance evaluation parameters are briefly discussed. Some reactor-based BNCT centers still treat patients in Argentina, China, Japan, and Taiwan. This will continue to be a major contribution to the further development of the BNCT.
Accelerator-based BNCT resources, already built or being installed, will certainly play an important role in the successful dissemination of BNCT as a standard cancer treatment. But fission reactors have so far provided a unique opportunity to demonstrate the feasibility and effectiveness of BNCT in many techniques that would be useful for accelerator-based BNCT. In addition, nuclear reactors can be very useful in continuing BNCT research to develop instrumentation, measure boron concentrations in new boron compounds, and in vitro and in vivo experiments. Another aspect of biomedical importance is the amount of exposure and the dose absorbed independent of the nature of the radiation. Biological damage mainly depends on the radiation energy received by the body’s materials. Energy losses for different types of radiation are significantly different. Evaluation of the biological effects of different types of radiation, dose equivalent to H dose has been introduced as a new experimental unit. The quality factor strongly depends on the ionization power of different types of radiation along the path. Different organs of the body respond differently to radiation. To determine the specific sensitivity to radiation exposure, the specific organ weight ratio of WT tissue is defined to expose a particular organ or tissue to a specific temperature. More information about this source text for more translation information, the source text is required.
Advantages of the Technique
1. They are cheaper and smaller.
2. They are safer and have less gamma contamination.
3. The neutrons produced are often low energy.
4. They have a more limited neutron energy spectrum.
5. They are easier to make.
6. Can be installed in or near a hospital.
7. They are adjustable and controllable.
Cooling System
Due to the linear energy drop of protons in beryllium, a lot of heat will be generated on its surface. Number of protons in 1mA current:
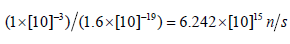



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.