Prospects of Perineural Implantation of Stem Cells in Recovery of Neural Networks' Functions in Brain Diseases
Abstract
Background: The question on prospects of additional use of
cellular therapy in standard protocols of brain neural networks recovery
after neurodestructive processes of various etiology was analyzed. Data
on low efficiency of existing methods of neurodestructive processes
treatment gave a boost to authors to conduct series of studies related
to brain diseases and cellular technologies. Existing treatment
principles now should be updated with new methods - in particular,
cellular technologies. This statement was based on stem cells (SC)
presence in human brain. Nature has formed basis for neural network
formation in the process of training and for recovery therapy in case of
pathology. Unfortunately, recovery potential of brain SC appeared to be
ineffective in neurodestructive processes. Therefore, SC should be
additionally injected into brain. It is advisable to use autologous SC
in order to avoid possible side effects, such as malignization of
allogeneic and other SC. But in certain cases, use of allogeneic SC can
be approved.
Methods: Autologous mesenchymal SC (MSC) are usually
administered to patients in experimental and clinical conditions. Two
ways of MSC delivery to brain are preferred: with cerebral blood flow
after MSC injection into bloodstream and MSC implantation close to
damaged area after additional skull trepanation. Authors experimentally
developed and clinically evaluated new technique of perineural
implantation of MSC. This method is based on natural ability of SC to
migrate. MSC are implanted into the area of cranial nerve endings
(mainly olfactory and trigeminal) and then they migrate along cranial
nerves to cranial cavity.
Results: The area of vomeronasal complex (VC) is preferred for
MSC implantation because its mucosa has nerve endings of both olfactory
and trigeminal nerves. Such technique of MSC implantation was
substantiated by authors and was named perineural implantation of MSC
when used in neurodestructive processes.
Discussion and Conclusion: The article contains critical
analysis and prospects of cellular technologies development for therapy
of brain diseases. Special attention is paid in discussion to technology
of perineural implantation of MSC in experimental and clinical
conditions.
Abbreviations: SC: Stem Cells; MSC: Mesenchymal Stem Cells; VC: Vomeronasal Complex
Introduction
Search for various logical combinations for SC and brain diseases in
PubMed on October 19, 2018 showed 29934 articles about "brain injury
cerebrovascular diseases", 764 about "brain injury cerebrovascular
diseases stem cells", 414 about "brain injury cerebrovascular diseases
stem cells human" and 4 - about "brain injury cerebrovascular diseases
stem cells human intranasal"Surprisingly, but these four articles
contain information about administration of human SC to experimental
animals, but not about therapy of human brain diseases. Authors of one
article [1] used human MSC taken from umbilical cord of newborn babies
for intranasal application in rodents with previously simulated hypoxia
or brain ischemia. It was established that intranasal administration of
human embryonic neural SC to neonatal rats with signs of encephalopathy
is accompanied with more effective and rapid recovery of behavioral
reactions control [2]. Intranasal injection of MSC from umbilical cord
to Wistar rats is accompanied with activation of microglia and
astrocytes leading to appearance of reparative properties of
oligodendrocytes [3]. This process is also accompanied with acceleration
of myelinization of neuron processes and optimization of interneuronal
communications together with reduction of gliosis zone in damaged brain
regions [3]. The range of mechanisms of positive effects of C3a peptide
after its intranasal application to rodents was specified [4].
Therefore, there are no articles in PubMed related to intranasal
administration of autologous SC for therapy of patients with brain
diseases.
Authors started conducting studies for assessment of SC perineural
implantation significance in activation of reparative processes about 10
years ago [5-12]. Patterns of SC migration in brain tissue have been
established in the experiments - depending on the area of SC injection
at cranial nerve peripheral endings and localization of neurodestruction
site in brain tissue [5,10-12]. Such principle of migration was
determined as somatotopic distribution of SC in brain [12].
SC consistently move directly to the site of destruction in brain
[5-12]. It is already proved nowadays that such aimed migration is
determined by various signaling molecules which are expressed by nerve
and glial cells in the area of neurodestruction [5-12]. Somatotopic
distribution of implanted SC in brain depending on the site of injection
is another one regularity [12]. In particular, implantation of SC into
the area of olfactory nerve endings results in SC distribution mainly in
anterior and middle cranial fossae. Implantation of SC into the area of
trigeminal nerve endings results in SC accumulation in posterior
cranial fossa [12]. Additionally, significance of the amount of
implanted SC was experimentally verified: it should be from several tens
of thousands up to millions per one ml of culture medium. It was also
experimentally stated that the best reparative results are reached when
SC are implanted during first hours and days of neurodestruction
development [8,11,12].
Modern Technologies of Brain Diseases Therapy
World statistics characterizes low efficiency of diagnostics and
treatment of acute and chronic brain diseases [13,14]. The situation
initiates search for new more effective technologies to resolve socially
important issues affecting aspects of life and capability of citizens
around the world. About six million people die due to stroke with
various parts of blood vessels involved [13] each year according to WHO
[13,14]. About 10 million people die each year due to brain trauma.
Surgery remains one of the key methods in treatment of such fatal
cerebral diseases as stroke, brain trauma, cerebral aneurysms and
neoplasms. Effectiveness of surgery really increases with implementation
of robotic devices (da Vinci Surgical System, Spine Assist, Renaissance
Robotic Systems), high- tech operations (micro-, endovascular and
stereotaxic surgery), combined therapy and new methods of rehabilitation
[13], creation of electronic and other devices, some of them have
already helped Stephen Hawking realize his unique intellectual
dispositions. Possibilities of diagnostic procedures in cerebral
diseases also enhance due to modern electronic equipment (CT, MRI, PET).
These technologies improve diagnostics at early stages of brain
diseases, but, unfortunately, don't improve treatment results [13].
All the countries constantly perform search for new more effective
ways of brain diseases treatment. And these methods have been already
developed in the fields of cellular biology and neurophysiology. For
example, cellular therapy was successfully adapted for treatment of
socially important diseases [8,9,11-14]. There were both followers and
opponents of their implementation in clinical practice - this usually
happens when elements of novelty appear in science and technics. Authors
overcame that stage and moved from experimental studies to clinical
implementation. This refers to technique of perineural implantation of
SC [11,12], which has set of advantages compared to traditional systemic
applications of SC (intravenous, intraarterial and intrathecal)
[13-19]. Enthusiasts mastered hard period of substantiation of prospects
of cellular technologies use in therapy of brain diseases. In fact, we
have new stage nowadays when experimental studies are rapidly followed
by implementation of new methods to clinical practice. There are both
encouraging [11,15,17-19] and negative [8,12,16,20] results of cellular
technologies use by doctors. It was found that intravenously and
intraarterially implanted SC have extremely low ability to penetrate
through blood-brain barrier to brain tissue [15,18]. There is another
complication after intrathecal injection of SC: cells are unable to cope
with craniocaudal flow of liquor [12]. Neurosurgeons perform SC
implantation directly to brain tissue, but these manipulations are
associated with additional surgical intervention (skull trepanation)
which is undesirable in acute period of disease development [17]. The
technique of perineural MSC migration of MSC to brain appeared to be an
original way out [7,8,11,12,20,21] in contrast to traditional surgical
interventions. Enhancement of this technique allowed developing
somatotopic method of MSC implantation followed by their aimed migration
to certain brain region [8,12] together with ways of visualization of
migration process in clinical conditions [22-25]. Implantation of MSC
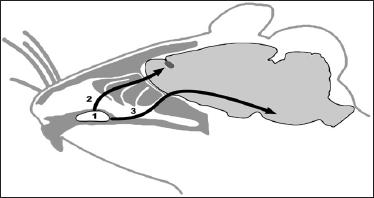
was performed into the VC area (Figure 1) from where MSC migrate to
region of neurodestruction by both olfactory and trigeminal nerve
endings [22-25].
Figure 1: Hypothetic ways of SC migration from VC of rat (1)
along olfactory (2) or trigeminal (3) nerves to cranial cavity (Figure
by Dmitry Tokalchik).
Material and Methods
Technique of MSC implantation into nasal submucosa was chosen due to
minimization of side effects and simplicity of accomplishment by
neurosurgeon [8,12,20-22]. Migratory abilities of SC were taken into
account during technique development [5,6,8,9,11,12] . Moreover, the
presence of endogenous SC in nasal mucosa says for MSC implantation
namely in that receptive field [8-11]. Considering localization of
central structures of olfactory analyzer in anterior cranial fossa, it
was proposed that the bulk of implanted SC will be located namely in the
structures of anterior cranial fossa.
Adipose tissue was previously separated from epiploon of anesthetized
rats, fermented with trypsin and plated in plastic flasks with culture
medium and gentamycin. MSC were painted for monoclonal antibodies to
CD90 (FITC- or PKH67-labeled) at the day of implantation. Brain slices
8|im in thickness were prepared and examined with fluorescent microscope
Zeiss Axio Vert 200M at the final stage. Concentration of MSC in
experiments on rats was 50 thousand cells per 50|il of culture medium.
Concentration of MSC for intranasal implantation in patients was from 6
to 10 million cells per 1ml of culture medium.
Results
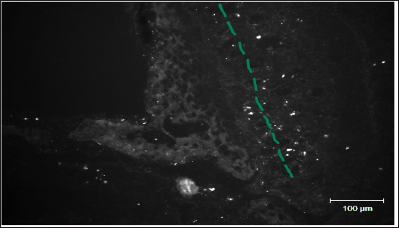
Figure 2 shows distribution of FITC-labeled MSC in the area of lamina
cribrosa ethmoid bone of rat after preliminary local destruction of
brain in sensorimotor zone and subsequent implantation of 50 thousand
MSC in 50|il of culture medium in the VC region.
Figure 2: A line represents mesenchymal stem cells migration pathways in the area of lamina cribrosa ethmoid bone of rat (Figure by Yuliya Takalchyk-Stukach).
Considering Patients in Clinical Conditions
It is advisable to inject MSC suspension into soft tissues of upper
and partially middle nasal conchae due to high density of olfactory
nerve endings [11,12]. By the way, we have previously demonstrated that
perineural implantation of SC is accompanied with activation of
reparative potential of endogenous SC [8,12]. Endogenous pools of SC are
located in three brain regions: area of olfactory bulbs, hippocampus
and brain regions close to ventricles [12]. Unfortunately, endogenous SC
lack reparative potential to recover impaired functions of nervous
system when neurodestructive processes, Parkinson's and Alzheimer's
diseases develop. Cellular technologies can help in these cases, namely
perineural implantation of autologous SC from adipose tissue of the
patient. These SC can migrate along nerve fibers from periphery to
neural networks in brain and spinal cord. SC express various trophic
factors on the way of migration, which have positive impact on both
functions of neurons and glial cells close to brain trauma and activity
of endogenous brain SC [8].
Unfortunately, authors understand that cellular technologies are not
always act as panacea - regarding the situation with Stephen Hawking
mentioned above. We lack more effective treatment approaches - for
example, the ones based on combination of cellular methods with
technologies of neural network formation in 3D-space. But this refers to
close future.
Conclusion
Development Prospects of Therapeutic Technologies in Brain Diseases
There are frequent situations in modern clinical practice which
require conditions for penetration of medicinal substances or SC into
brain. Selective permeability of blood-brain barrier prevents
penetration ofcellular elements into cranial cavity (SC, in particular).
At the same time, protocols of systemic administration of MSC have been
recently developed for treatment of neurodestructive processes. Above
mentioned stimulated authors to develop an alternative method which
would allow increasing effectiveness of cellular elements and medicinal
substances penetration into cranial cavity. It is also necessary to
develop such technology which will guarantee advanced penetration of SC
but not toxic substances into brain tissue. Scientists who are aimed at
widening of cellular technologies area of stem cells use should set
sights on solving of mentioned problem and enhancement of developed
technology.
Accuracy of Distal Long Femur Nail Locking with Different Techniques - https://biomedres01.blogspot.com/p/blog-page_21.html



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.