Voltammetric Determination of Anthrone Using Cetyl Trimethyl Ammonium Bromide Surfactant Modified Carbon Paste Electrode
Introduction
Anthrone is an aromatic ketone [1]. It is widely used as in the colorometric determination of carbohydrates, and it is widely used as a laxative. ANT can cause respiratory irritation; long term exposure may result in disease of airways involving difficult breathing and related systematic problems. Over the last decades biosensor related research has shown a dramatic growth. The action of surfactants in electroanalytical field has been widely reported [2-18] and was widely used which will increase the electron transfer and also improve the detection limit of biologically active molecule. With increasing the use of ANT, it became an important to develop a simple, reliable, sensitive and simple cost analytical method for the determination of ANT. During the past decades’ scientists are focused to developing Voltammetric methods for the detection of electroactive species. Cyclic voltammetry [19-21] is a simple, powerful, widely used method in electrochemical studies, and which has been widely used to investigate the electrochemical properties of the species which are electroactive in nature. After invention of many of the new material a need has been expressed for developing of stable simple and efficient material as sensors for the determination of electroactive species by electrochemical method. At present, whole area of research occupied with developing of new material and fabrication of new electrochemical sensors.
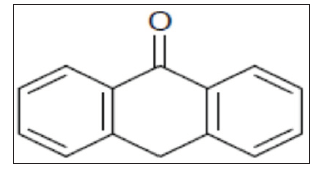
Chemically modified electrode [22] has got an important application in medicine and pharmaceutical analysis. In electroanalytical research determination of electroactive species by voltammeric method become the major target. Carbon Paste Electrode (CPE) [23] has been widely used as working electrode in the electrochemical application because of simple preparation, easy renewal, low cost and is more compatible with biological tissues. Because of all these advantages of CPE, become the suitable substrate for the preparation of modified electrodes. In the present work, we developed a suitable sensitive and selective chemically modified electrode for determination of ANT. Here, we describe CV and Differential Pulse Voltammetry (DPV) studies of ANT. There is no work has reported on the determination of ANT by Cetyl Trimethyl Ammonium Bromide Modified Carbon Paste Electrode (CTABMCPE) and the structure (ANT) is shown in Figure 1.
Materials and Methods
Chemicals
CTAB was purchased from Molychem India Pvt. Ltd. Graphite, Silicone oil, Anthrone was obtained from Nice Chemicals, India and stock solution of ANT was (25×10-4 M) was prepared in acetone. 25×10-3 M CTAB was prepared in distilled water. Monosodium hydrogen phosphate and disodium hydrogen phosphate was received from Merck specialties Pvt. Ltd. Phosphate buffer solution was prepared by mixing 0.2 M Na2HPO4 and 0.2 M NaH2PO4 and buffer solution having different pH values prepared by mixing appropriate amount of Na2HPO4 and NaH2PO4. All the experiments are carried out under room temperature (25 ± 1 °C) and distilled water was used to prepare all the solution.
Electrode Preparation
BCPE was prepared as mixing 70% graphite powder and 30% silicone oil in agate mortar for about 20 minute to get a homogeneous paste. This carbon paste was packed in to a teflon tube cavity having 3mm diameter. The electrochemical contact was setup with a copper wire contact to the paste. Before placing the electrode for measurements, it was smoothed on a tissue paper to get uniform and smooth surface. CTABMCPE was made by immobilizing 10 μL CTAB on the surface of the electrode for 5 minute.
Instrumentation
All the electrochemical experiment was carried out using a potentostat (EA-201 electroanalyser, Mumbai, India). A conventional three - electrode set up with the CTABMCPE and BCPE as working electrode, Pt wire as counter electrode and saturated calomel electrode (SCE) as reference electrode was used for the electrochemical measurements. A personal computer was used for data storage and processing. All the potential is measured versus the SCE.
Result and Discussion
Effect of CTAB Concentration
Figure 2: Cyclic voltammogram of 2×10-4 M ANT at BCPE with different concentration of CTAB, 5 μL to 20 μL in 0.2 M PBS (6.5 pH) at the scan rate of 100 mV/s, (b) Oxidation peak current Vs CTAB concentration.
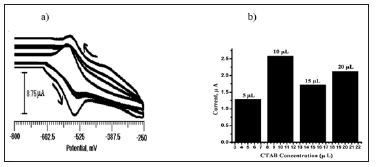
Figure 2a shows the relationship between concentration (5 μL, 10 μL, 15 μL, 20 μL) of CTAB and peak current of ANT. CTAB exhibits a remarkable enhancement of peak current of ANT. Peak current has increased by increasing the concentration of CTAB up to 10 μL but after 10 μL it has observed that the peak current was decreasing. The amount of CTAB modifier was optimized as 10μL for the best voltammogram and was chosen for the experiment. The dependence of the oxidation peak current of ANT on different concentration were drawn and shown in Figure 2b.
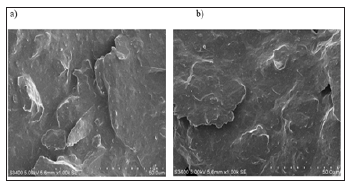
Morphology Studies
BCPE and CTABMCPE were characterized by VPSEM. Figure 3 shows the typical VPSEM image of BCPE (a) and CTABMCPE (b). The surface of BCPE was irregularly arranged with the graphite. But it can be seen that there is a uniform arrangement of Surfactant molecule CTAB on the surface of the carbon paste electrode. From the VPSEM image it is clear that the carbon paste electrode was coated by surfactant, which will lead to improve the surface properties CTABMCPE.
Electrochemistry of ANT
Figure 4: Cyclic voltammogram of CTABMCPE with ANT (2×10-4 M) (dashed line) and without ANT (solid line) in 0.2 M PBS, pH 6.5 at the scan rate of 100 mV/s.
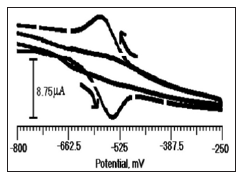
In order to know the electrocatalytic activity of ANT on CTABMCPE, cyclic voltammetric response were obtained at 0.2 M PBS (pH 6.5) in the absence (solid line) and presence (dashed line) of 2×10-4 M in the potential range -800 mV to -250 mV, and data were shown in Figure 4. There is no redox peak was observed in the absence of ANT, and there is a drastic enhancement of anodic peak current (Epa = -543 mV and Ipa = -2.59 μA) and cathodic peak (Epc = -575 mV and Ipc = 12.77 μA) obtained by the addition of 2×10-4 M ANT which indicates that the electrochemical response of ANT improved at CTABMCPE in other word electrocatalytic activity of ANT has increased at CTABMCPE.
Voltammetric Behavior of ANT at CTABMCPE
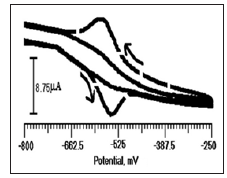
Figure 5 shows the cyclic voltammogram of ANT (2×10-4 M) at CTABMCPE (dashed line) and BCPE (Solid line) at 0.2M PBS (pH 6.5). A well-defined redox peak was observed for ANT at CTABMCPE (-543 mV (Epa) and -575 mV (Epc)), compared with BCPE (no peak was observed). On this observation, it is clear that surfactant improves the catalytic effect of ANT, which will lead to the enhancement of peak current. The peak current obtained for CTABMCPE is much larger than compared to BCPE.
Figure 5: Cyclic voltammogram of ANT (2×10-4 M) in 0.2 M PBS buffer solution of pH 6.5 at BCPE (solid line) and CTABMCPE (dashed line).
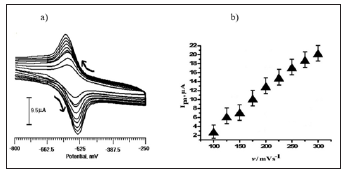
Effect of Scan Rate on Peak Current of ANT
The effect of potential scan rate on peak current of ANT at CTABMCPE at 0.2 M PBS (pH 6.5) was investigated in order to study the reaction mechanism. Figure 6a shows the effect of scan rate and peak current relation in the range of 100 mV/s to 300 mV/s. It has been seen that the peak current linearly increased with the increase of the scan rate and shows the electrode process was adsorptioncontrolled process, with s linear equation of Ipa= -5.59+ 0.088 v (mV/s) with a correlation coefficient (R) of 0.99534 (Figure 6b).
Figure 6: Note:
a) Cyclic voltammogram of ANT (2×10-4 M) at CTABMCPE in pH 6.5 at various scan rates from 100 to 300 mV/s
b) Plot of peak current of ANT as a function of scan rate.
Effect of pH on Peak Current of ANT
The effect of pH on the CTABMCPE for the determination of ANT was investigated in the range 6.0 to 8.0 using 0.2 M PBS at the scan rate of 100 mV/s by CV (Figure 7a). It was observed that with increase of pH the peak current was shifted to less negative direction and maximum value obtained at pH 6.5, in order to get good sensitivity and selectivity, 6.5 pH was taken as optimum value (Figure 7b). The plot of anodic peak potential Vs pH (Figure 7c) exhibited a linear relationship with a regression equation of, Epa (mV) = 160.6+59.6 pH with a correlation coefficient of 0.9976 From the slope 59.6 mV/pH it is clear that the value is close to the theoretical value 59 mV/pH shows that number of protons and electrons involved in the reaction of ANT oxidation are equal.
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.