Metal Ion Binding of The Corynebacterium pseudotuberculosis Diphtheria Toxin Repressor
Introduction
The acquisition of essential nutrients and ions such as iron is of primordial significance for the growth and development of bacterial pathogens [1]. Since the concentration of free iron in human serum is estimated to be 10-12 μM, which is much lower than that required for optimal bacterial growth [2], invasive pathogenic bacteria overcome this problem by using inducible, high-affinity iron uptake systems, siderophores, to scavenge iron from the host [3,4]. In gram-positive and gram-negative bacteria, the diphtheriae toxin repressor (DtxR) plays a key role in the regulation and production of siderophores and genes involved in the uptake of iron and other transition metals [5-10]. Additionally, DtxR regulates the expression of diphtheriae toxin in C. diphtheriae [11]. The threedimensional structure of C. diphtheriae DtxR (Cd-DtxR) reveals that it exists as a dimer containing two metal binding sites per monomer [12,13]. Several transition metal ions, such as Fe(II), Ni(II), Co(II), Cd(II), Mn(II) and, to some extent, Zn(II) activate apo-Cd-DtxR and, the holo-enzyme binds its target DNA and blocks the transcription of the downstream genes [14-17]. Yellaboina et al. [6] predicted more than 70 DtxR-regulated operons in the C. diphtheriae genome and demonstrated that DtxR is a global regulator of cell metabolism.
Our study was performed on DtxR of Corynebacterium pseudotuberculosis, a related pathogen of Corynebacterium diphtheriae. Analysis of the C. pseudotuberculosis genome identified diphtheriae toxin and DtxR genes. However, diphtheriae toxin is not involved in the C. pseudotuberculosis infection. We assume that DtxR in C. pseudotuberculosis mainly plays a role in divalent ion uptake and in the regulation of metabolism. C. pseudotuberculosis causes caseous lymphadenitis (CLA) in equids, sheep, goats, and to a lesser extent in horses and cattle. This disease leads to considerable economic loss in many countries, including Brazil [18,19] and presently, no effective treatment is available to combat this disease. Based on its key role in pathogen cell metabolism, Cp-DtxR can be considered as a potential drug target and we present results of the expression, purification and characterization of Cp-DtxR and the iron binding by Cp-DtxR apo-protein.
Material and Methods
In Silico Analysis: Cp-DtxR and Cd-DtxR sequences were retrieved from NCBI and a sequence alignment were performed using MUSCLE [20] and Box Shade web servers. The atomic coordinates from C. diphtheriae DtxR (PDB code: 1G3S) were used as the template for comparative modeling by the satisfaction of spatial restraints as implemented in the program Modeller 9v13 [21]. The structure of native Cp-DtxR (Gene ID: ADL10687.1; Uniprot: D9QAW1) was modeled.
Expression and Purification of DtxR: The open reading frame of Cp-DtxR was cloned into the vector pD441-SR by DNA 2.0 (USA). The construct contains an N-terminal TEV cleavage site and hexahistidine affinity tag. The kanamycin-resistant vector pD441- SR presents a T7 promoter inducible by IPTG and a high copy percentage provided by pUC origin of replication. DtxR-pD441-SR (DNA 2.0) vectors were transformed into E. coli BL21 (DE3)T1 (Sigma-Aldrich, USA) competent cells, which were grown for 16 h (overnight) at 37°C in LB medium containing sufficient amounts of kanamycin. The bacterial cultures were transferred into fresh LB medium, grown for another 3.0 h at 30°C until the OD600 reached 0.6. Subsequently, Cp-DtxR expression was induced by 0.4 mM IPTG, and incubated for 4 h at 30°C, being supplemented with 50 μM FeCl2. Later, the culture was harvested by centrifugation at 4000 g, 5°C for 20 min, discarding the supernatant and re-suspending the Cp-DtxR containing cell pellet in 20 mM K2HPO4/KH2PO4 pH 7.4, 500 mM NaCl, 5% (v/v) glycerol, 2 mM imidazole.
The cell-suspension was incubated on ice for 1 h with lysozyme, subsequently being lysed by sonication in four sets of 30 s pulses of 30% amplitude, with 10 s intervals. This way obtained crude cell extract was centrifuged at 8000 g, 6°C for 90 min. The supernatant containing Cp-DtxR was loaded onto Ni-NTA column preequilibrated with 20 mM K2HPO4/KH2PO4 pH 7.4, 500 mM NaCl, 5% (v/v) glycerol, 2 mM imidazole, extensively washed with the same buffer containing 20 and 60 mM imidazole, Cp-DtxR eluted with 300 mM imidazole. The eluted fractions were individually pooled and injected onto a Superdex 75 10/300 GL size exclusion column (GE Healthcare), pre-equilibrated with buffer 20 mM K2HPO4/ KH2PO4 pH 7.4, 150 mM NaCl. Sample purity after each purification step was assessed by 15% SDS-PAGE gels. Fractions containing Cp-DtxR were concentrated in a micro concentrator (MWCO: 3000Da, GE Healthcare). Protein concentrations were determined spectrophotometrically, applying the Lambert-Beer law [22].
CD Spectroscopy:
For all CD measurements, 15 repeated scans were performed, 5 scans were used to establish the baseline. The wavelength range applied for far-UV spectra was from 200 nm to 260 nm, in a time constant of 1 s and 100 nm/min continuous scanning mode, using a Jasco J-107 spectropolarimeter (Jasco, Japan). Prior to conducting the CD experiments, Cp-DtxR was incubated overnight with 1 mM EDTA, to remove irons from the specific binding sites. EDTA and EDTA-ion complexes were eliminated by dialysis against 20 mM K2HPO4/KH2PO4 pH 7.0. Cp-DtxR was diluted to a concentration of 1 μM. The effect of the iron ions was tested on the protein secondary structure. The protein was pre-incubated with 10 μM Fe2+ for one hour prior to the measurements. The results are presented in molar ellipticity [θ], according to: in which, θ is the ellipticity measured at a given wavelength λ (deg), c is the protein concentration (mol L−1), l is the cell path length (cm) and n is the number of amino acids. CDpro software package was adopted to analyze the results [23].
Results and Discussion
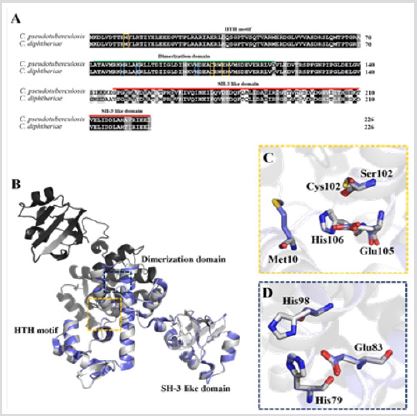
Sequence Alignment and Molecular Model of Cp- DtxR: A BLAST search for the Cp-DtxR sequence against the nonredundant protein sequences database [24] indicated that the protein sequence is highly conserved within the Corynebacterium genus (75% to 95%) (Figure 1A). A Blast search for the Cp-DtxR sequence against the data deposited with the Protein Data Bank showed a sequence identity of 78% with the Cd-DtxR protein structure (PDB: 1G3S), which was used to generate a Cp-DtxR model. The DtxR proteins are dimers and contain three highly conserved domains important for the protein function, which is characteristic of the protein family [12,13]. The HTH motif is important for DNA binding. The SH3-like domain shows more differences in the protein sequence (Figures 1A & 1B); however, the residues coordinating the metal binding sites 1 and 2 are conserved within the two species (Figures 1A-1D). The structural overlay of the Cp-DtxR model and the Cd-DtxR crystal structure resulted in an rmsd value of 0.135 (1433 atoms) and indicates the significant conservation of the protein structure within the Corynebacterium family.
Figure 1: Sequence alignment and structural comparison of Cp-DtxR and Cd-DtxR.
b. Structural comparison of Cp-DtxR homology model (in grey) as dimer and Cd-DtxR crystal structure (PDB: 1G3S in blue). The domains are shown and the both ion-binding sites in the monomer are highlighted.
c. Amino acids coordinating metal binding site 1.
d. Amino acids coordinating metal binding site 2.
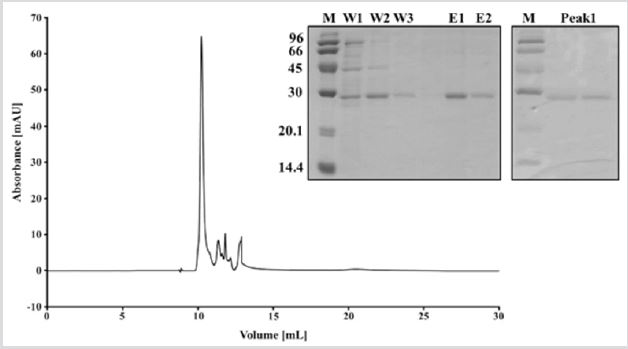
Protein production and purification: The Cp-DtxR construct consists of 239 amino acids (including TEV cleavage site and hexa-histidine tag) with a molecular weight of 26.877 Da. The protein presented a single band on a denaturing SDS-PAGE gel (Figure 2) with an apparent molecular mass of approximately 30.000 Da, after the two-step purification (Figure 2). The size exclusion chromatography demonstrated that the protein exists in solution as a dimer, which was confirmed with a SEC calibration (supplementary Figure 1).
Figure 2: Purification of Cp-DtxR. SDS-PAGE analysis of Cp-DtxR after Ni-NTA purification. M: protein marker,W1-3: washing steps, E1-2: elution steps.SDS-PAGE of Cp-DtxR after size exclusion chromatography, which indicate the purity of the protein. Chromatogram of size exclusion chromatography of Cp-DtxR, elute at around 10.5 ml, which corresponds to a dimer of Cp- DtxR.
CD spectroscopy: Protein conformational changes induced by iron ions were monitored using far-UV CD spectroscopy. Thereby the effect of Fe2+ binding to apo-Cp-DtxR were investigated (Figure 3). The results of the secondary structure prediction of Cp-DtxR by CDpro [23] based on the results of the far-UV CD measurements indicated 34% α-helices, 61% random coiled regions and 5% β-sheet in the protein secondary structure. The results indicated a high α-helical and random coiled content in the secondary of the protein, these results are in agreement with the existing 3D structures of DtxR proteins for example from C. diphtheriae(PDB: 1G3S), 43% α-helices and 14% β-sheet. Secondary structural change due to Fe2+ binding was observed and analyzed using the CDpro software [23]. The results demonstrated that following Fe2+ binding, an increase in the α-helical content (34% to 38%) of the protein secondary structure is observed. Local structural rearrangements following metal ion binding to apo-DtxR have been correlated with helix-to coil transitions [25], which are in close agreement with our CD results.
Figure 3: Conformational changes in the Cp-DtxR secondary structure studied by CD spectroscopy. A: CD spectra of Cp- DtxR incubated with EDTA. B: Overlay of the CD-spectra from Cp-DtxR incubated with EDTA and after treated with Fe2+.
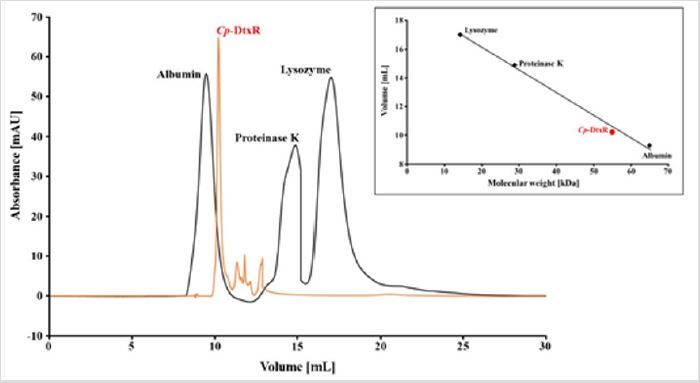
Figure S1: Calibration of the SEC column used for the Cp-DtxR purification. The chromatogram show the elution peaks of Albumin (65 kDa), Proteinase K (29.7 kDa) and Lysozyme (14.3 kDa). Cp-DtxR elute at around 10.5 ml, the protein monomer has a MW of around 27 kDa, but the protein elute as dimer, near a MW of 60 kDa as demonstrated in the linear elution plot.
Conclusion
The diphtheriae toxin repressor from C. diphtheriae is an intensively investigated cellular protein. The protein controls diphtheriae toxin production and the production of siderophores and is a global regulator of the cell metabolism. C. pseudotuberculosis is a related pathogen of C. diphtheriae and contains the diphtheriae toxin and DtxR genes, but diphtheriae toxin is not considered to be directly involved in the infection mechanism of C. pseudotuberculosis rather, DtxR seems to play regulatory function in the transition metal uptake and the cell metabolism. The function and importance of C. pseudotuberculosis DtxR remains poorly understood and research in this area can contribute to narrow the search for molecular targets against caseous lymphadenitis.Our results describe for the first time a protocol for the heterologous gene expression of Cp-DtxR in E.coli cells and a subsequent two-step purification process. The production of protein in a high amount and quality are necessary for further investigations of Cp-DtxR.
Formation of Precipitates on the Surface of Root Dentin After Various Final Irrigation Protocols-https://biomedres01.blogspot.com/2021/02/formation-of-precipitates-on-surface-of.html
More BJSTR Articles : https://biomedres01.blogspot.com




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.