Mini Review: Exosomes from Discovery to Isolation
Introduction
Exosomes were described for the first time over thirty years ago in 1983 [1]. Two papers, that were released within a week of each other, both showed that reticulocytes’ transferrin receptors were externalised into the extracellular space [2,3]. Harding, Heuser and Stahl showed for the first time that a type of late endosome dubbed “multivesicular endosomes” or MVEs was responsible for the recycling of the transferrin receptor. To their surprise, they found that the MVEs fused with the plasma membrane which led to the release of small vesicles, under 100nm in diameter, found within MVEs [4] (Figure 1).
Figure 1: Exocytosis of MVEs and release of exosomes. Electron microscopy images of quick-frozen fixed reticulocytes labelled with AuTf showing A. fusion of MVE membrane with the plasma membrane B. and the release of transferrin receptor loaded exosomes into the extracellular space. Bar 100nm, Harding 1983.
At the same time, Pan and Johnstone proved in sheep reticulocytes that the transferrin receptor is externalized via vesicles. Although they did not visualize the phenomenon by electronic microscopy, they used anti-transferrin-receptor antibodies to follow the trafficking of the protein [3]. This discovery showed that plasma membrane recycling did not only occur for molecules bound to early endocytic compartments, as was the view at the time, but also molecules present on late endosomes such as MVEs.
Indeed, other molecules found deeper in the endocytic system were also recycled to the plasma membrane. For example, processed antigens seemed to be recycled from lysosomes to endosomes in order to bind to MHC-II. This complex is then expressed on the cell surface for T cell presentation [5]. Also, Lippincott-Schwartz and Fambrough found that the Lysosome-Associated Membrane Protein (LAM]P-1), then named LEP 100, was addresses to the plasma membrane [6].
Exosomes are secreted by virtually all cell types, but most notably by immune cells. Their immunomodulatory functions were first demonstrated by Raposo in B cells. They found that MHC-II-enriched MEVs released exosomes that could present MHCII-peptide complexes to T cells and induce an immune response [7]. Knowing this, other studies then looked at dendritic cells and found they could also secrete T-cell activating exosomes that are potent enough to eradicate tumours in an in vivo mouse model [8- 10]. However, dendritic cell-Derived Exosomes (DEX) seem to be the only ones capable of activated naive T cells [11]. It was further described that naïve CD4+ T cells need the presence of Antigen Presenting Cells (APCs) for this activation to take place, leading us to believe the exosomes are bound to the APCs membrane for this [12,13]. But a potent activation of naïve CD8+ T cell is found in the absence of APC but stimulated by IL-12 [14,15].
Moreover, macrophage-derived exosomes can also present antigens to T cells in a cell-surface-bound exosomes system [16]. It is noteworthy to mention that in this study, the cells were infected with Mycobacterium Tuberculosis (Mtb) and this alone enhances the production of exosomes. It has also been described in many virus-associated pathologies that exosomes favour the expansion of viral infection. For instance, HIV has been known to hijack the exosomal packing and/or release pathways to its advantage [17]. It has been suggested that HIV can charge its viral RNA into the infected cells’ exosomes and intensify their release to facilitate the infection of healthy neighbouring cells.
Biogenesis
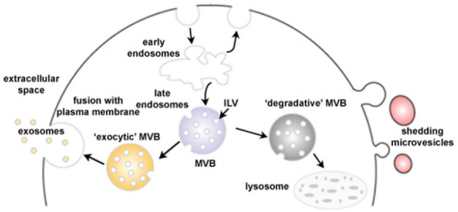
Exosomes differ to other secreted vesicles notably by their biogenesis. They arise in late endosomal compartment MEVs dubbed Multivesicular Bodies (MVBs) and when not-yet secreted are referred to as Intraluminal Vesicles (ILVs). Although unknown for a long time after their discovery, new light has been shed on exosome formation [18]. Molecules that are found on MVB membrane are either degraded after fusion of the MVB with lysosomes or secreted in the extracellular compartment after fusion with the plasma membrane [19,20] (Figure 2). In 2001, a paper by Katzmann showed that the sorting of ubiquitinylated MVB cargo was dependant on a 350kDa complex dubbed Endosomal Sorting Complex Required for Transport-I (ESCRT-I) [21,22]. Previous work identified ESCRT proteins as the major player in protein sorting of the vacuole in yeast [23]. The ESCRT machinery is made up of vacuolar protein sorting (Vps) subunits and 4 different protein complexes have been reported: ESCRT-0, I, II and III, but can also be associated to the AAA ATP Vps4 complex [24]. ESCRT is able to sequester and sort cargo and deform the MVEs membrane to bud inwards to generate MVBs [25].
Figure 2: Schematic representation of exosome biogenesis. Early endosomes mature into late endosomes named MVBs in which ILVs have formed after inwards budding of the membrane. The MVBs have two possible fates: lysosome degradation by membrane fusion or release of the exosomes into the extracellular space by fusion with the plasma membrane (Adapted from Mathivanan 2010).
In the case of exosomes, elucidating the pathway leading to the fusion of the MVE membrane with the plasma membrane is key. Many proteins are involved in exosome biogenesis, from the inward budding of the MVE to the trafficking and subsequent fusion with the plasma membrane. The sphingolipid ceramide contributes to the transfer of specific exosome-associated cargo into the lumen of exosomes as well as their release. But has also been shown to trigger the curving of the vesicle membrane and subsequently bud [26,27]. It has also been suggested that calcium variations are important in the regulation of exosome biogenesis [28,29].
But other ESCRT-independent pathways of exosome biogenesis have been reported [30]. Ghossoub, found that the GTAPase ADP Ribosylation Factor 6 (ARF6) and Phospholipase D2 (PLD2) control exosome biogenesis via an ESCRT-independent pathway involving ALG-2 interacting protein X (Alix) [31]. Blocking Alix did not lead to a decreased secretion of exosomes but rather a change in their composition. Meaning that Alix is more likely to be involved in cargo loading. However, silencing of STAM1 or TSG101 led to no or partial reduction of exosome secretion but mainly to a variation in protein composition [25,32]. Hoshino even showed that an increase in exosomes secretion after Hrs knockdown, a member of the ESCRT-0 complex, lead to a more invasiveness cancerous behaviour [33].
The release of exosomes into the extracellular domain depends on the fusion of the MVE membrane with the plasma membrane [34]. For this, MVE docking involves multiple GTP-binding proteins such as Rab4, Rab5, Rab11, Rab27, Rab35 and ARF that are all involved in exosome secretion [35-38]. Other regulators include the cortical actin regulator (cortactin) 39 and the fusion regulator synaptotagmin-7 [33]. It has recently been suggested that exosome biogenesis is not necessarily ESCRT-dependant or independent but rather that the two pathways could act synergistically or be compensatory [40]. For example, Alix is known as an ESCRT accessory protein but is also involved in ESCRT-independent pathways. And a third pathway of exosome biogenesis involving the tetraspanin CD63 has been described as both ESCRT-dependant and independent [41]. The idea is that the pathway of exosome biogenesis defines the nature, composition and content of the exosome.
General Characteristics
Exosomes are nanovesicles of 30-120nm in diameter and are limited by a lipid bilayer. Unlike other Extracellular Vesicles (EVs), their origin is endocytic. As a result, exosomes are rich in endosomeassociated proteins such as tetraspanins, which are often the gold standard for exosome identification. Although other molecules such as heat shock proteins and MHC Class I and II are recognised as “exosome markers” they have also been found on other nonexosomal EVs in a study carried out on DEXs [42]. Much work has gone into deciphering the protein, lipid and RNA content as well as the functions of exosomes. Databases such as EVpedia, Vesiclepdia and Exocarta all contribute to understanding this.
Functions: Cell-to-Cell Communication
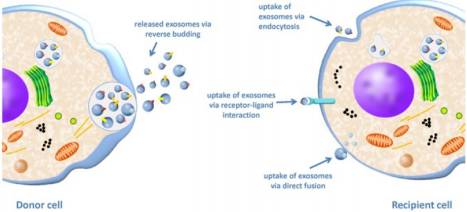
Exosomes can be considered as “mini cells” in that they have the same membrane orientation as the cell itself, RNA can be contained in their lumen, they carry ligands, receptors and other various signalling molecules. Many ways of communication with the target cell have been described. Exosomes can release their cargo in the extracellular space near the target cell, they can also be endocytosed, fuse with the plasma membrane, work in a “kiss and run” manner or simply release their content into the cells’ cytoplasm before targeting another cell [43] (Figure 3).
Figure 3: Exosome uptake from a donor cell to a recipient cell. The donor cell releases exosomes by fusion of the MVB membrane with the plasma membrane and subsequent secretion of ILVs into the extracellular space giving exosomes. The exosomes then interact with the recipient cell via three possible mechanisms: uptake by endocytosis, ligand/receptor interaction or by direct fusion of the exosome membrane with the plasma membrane [44].
Nevertheless, how the exosomes target recipient cells still remains largely unknown. We are only just scratching the surface but some studies are starting to decipher this [44-46]. Targeting could be led by either ligand-receptor affinity or by chemokines found on exosome. The latter would explain the recruitment of exosomes to more distant sites [47]. An interesting study by Heusermann also found that exosomes can bind to the active regions of filopodia and lamellipodia to then enter at endocytic hot spots of huh7 cells [48]. Moreover, this study demonstrated that exosomes taken up by the cell remain intact and are seemingly addressed to the Endoplasmic Reticulum (ER) where exosomal cargo is released before subsequent degradation by fusion with lysosomes. It has interestingly been discovered that siRNA and miRNA loading into RNA-silencing complex RISC takes place in the ER membrane [49- 51]. Thus, the authors put forward the hypothesis that this would allow an efficient entry of exosomal RNA into the cell’s translational machinery.
The study of miRNAs has been gaining interest as they are now seen as a potential biomarker for many diseases, including cancer [52]. Part of circulating miRNAs are found in exosomes [53] although other studies have found that most of miRNAs are linked to the Ago2 protein [54]. It was shown that miRNA enrichment on exosomes can be regulated by the oncogene KRAS [55] and the ribonucleoprotein A2B1 (hnRNPA2B1) [56]. Regardless, exosomes do carry miRNAs and these have potent biological effects ranging from regulating translational activity to stability and localisation in recipient cells [57].
Exosomes have the advantage of being stable in the extracellular media notably due to their lipid bilayer, unlike free single molecules that are more exposed to degradation. Moreover, exosomes can travel virtually anywhere in the organism, even passing through the cells and nucleic membrane via microtubes [58] and nanotubes [59].
Functions: Immune Modulation
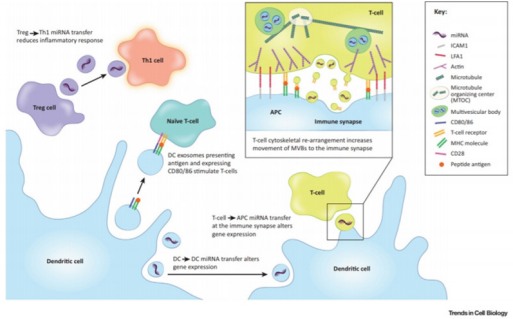
It is known that exosomes are secreted in larger amounts by immune cells compared to other cell types. Indeed, it was shown that exosomes alone can mediate antigen presentation and thus are capable of inducing a potent immune response [60,61] (Figure 4). MHCII αβ dimers found on the plasma membrane of APCs can be incorporated into ILVs. After this, the charging of the antigen onto the MHCII happens within the MVBs [62]. And the subsequent release of exosomes charged with MHC II-antigen complexes into the extracellular media leads to the activation of naïve CD4 T cells [11]. It was shown that the major release of these exosomes happens at the immunological synapse site between APCs and T cells. DCs can also induce a T cell response by releasing exosomes that express co-stimulatory molecules CD80 and CD86 or mDC intercellular Adhesion molecule-1 (ICAM-1) [11,63,64]. A study by Qazi, showed in vivo that antigen-charged exosomes alone can induce a potent Th1 response [65]. Thus, exosomes secreted at the immunological synapse site allow inter-cellular communication between the different immune cell populations. Indeed, DEXs are taken up by T cells but T cells can also release exosomes, containing miRNAs and/or TCR, that can regulate gene expression and cell signalling of DCs [66,67]. Monteclavo also found that mice DCs could exchange functional miRNA via exosomes [68] (Figure 4).
Figure 4: Immune modulation by exosomes. DEX carry miRNA, co-stimulatory molecules (CD80/86) and antigen charged MHC molecules that can be transferred to (i) naïve T cell to subsequently induce anti-tumour Th1 T cells, (ii) other DCs (iii) or T cells at the site of the immunological synapse to modulate the immune response. T cells can in return also secrete exosomes that will influence recipient DCs via miRNAs or the TCR that they carry. Exosomes derived from Tregs can block the immune response by hindering Th1 T cells via exosomal miRNA (Maas 2017).
Nevertheless, exosomes can also be used by regulatory immune cells to block the immune response and induce “infectious tolerance” [69]. In fact, mice Tregs were found to secrete exosomes that contain the miRNA let-7d that is taken up by CD4+ TNFγ+ Th1 cells and reduce their immunogenicity [70]. Other studies have shown that exosomes derived from mice Tregs can induce seemingly tolerogenic DCs via miR-150-155p and miR-142-143p [71]. However, the exosome-mediated tolerance of Tregs goes beyond miRNAs. Indeed, Okoye found that other factors such as non-coding RNA, chemokines, interleukins, collagen and matrix proteins found on mouse Treg-derived exosomes could induce immune tolerance (Figure 4). The immunosuppressive effect of Treg-derived exosomes seems so potent that it is even being considered as a possible therapeutic for autoimmune diseases and transplant patients [72,73].
Moreover, immunosuppressive Mesenchymal Stem Cells (MSCs) can use exosomes to inhibit macrophage response by impairing the recognition of Toll Like Receptors (TLR) [74] but also by triggering Treg differentiation after a TGF-β and IFNγ stimulation [75]. Other in vivo murine studies show that exosomes derived from tolerogenic DCs and Myeloid-Derived Suppressor Cells (MDSCs) favour Treg expansion and an inhibition of the Th1 response [76,77]. Although these results are very encouraging, we must bear in mind that most of these studies are carried out on mouse Treg-derived exosomes and further studies on human cells need to be done.
Thus, the use of exosomes in a systemic response such as the immune response makes perfect sense as they are highly itinerant and efficient. They most probably contribute to the fine-tuning of the immune balance in an energy-efficient and systemic manner.
Isolation Methods
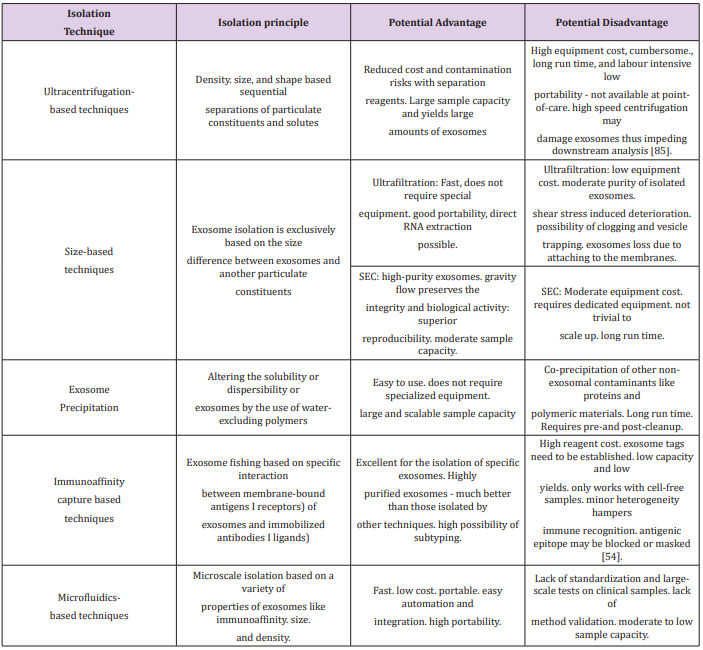
Exosomes can be isolated via two main methods: a series of high speed centrifugations with the use of a gradient (sucrose or iodixanol flotation) [78] or by immunoaffinity-based capture. Both methods have proven highly efficient and with a good yield [79,80]. However, it must be pointed out that the immunoaffinitybased capture can only be used for characterisation of exosomes as functional tests would be hindered by the binding agent [81,82]. And ultracentrifugation could also damage the exosomes by the strong pressure the exosomes are put under [83]. Other methods have also been developed such as exosome precipitation using waterexcluding polymers (polyethylene glycol) [84,85] or microfluidicsbased isolation [86,87]. The following table summarises the advantages and disadvantages of the above-mentioned techniques (Table I). Each method has its pros and cons, but what is essential is to develop a technique that will lead to widespread use of exosome, hopefully for the clinic. This must be a cost-effective and easily mastered isolation method.
More BJSTR Articles : https://biomedres01.blogspot.com/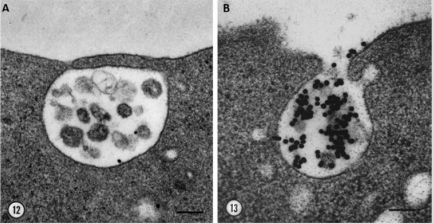



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.