The Protective Role of Silicon in Sugarcane Under Water Stress: Photosynthesis and Antioxidant Enzymes
Introduction
Silicon (Si) is not considered as an essential plant nutrient element, and yet numerous scientific reports have shown its positive role in many plant species and environment circumstances Coskun et al. [1]. Silicon is an agriculturally important fertilizer that enhances plant tolerance to environmental stresses Hodson et al. [2]; Epstein et al. [3-7]. Silicon increased leaf transpiration rate and consequently mediated the loss of photosynthetic parameters by water stress in plants Sonobe et al. [7]. In past studies, antioxidant activities were activated by Si element during drought in maize Kaya et al. [8] and wheat Gong et al. [9,10]. Plants benefit from Si fertilization and uptake of various plant species is carried out as a standard method in agricultural and horticultural crop plants Ma et al. [11]; Guntzer [12].
In plants, silicon is alleviated a wide range of environmental stresses such as radiation Shen et al. [13], lodging Savant et al. [14], wounding Kim et al. [15], heat temperature Muneer et al. [16], hypoxia Fleck et al. [17], soil salinity Flam-Shepherd et al. [18], water deficit Liu et al. [19], nutritional imbalance, i.e. Fe (Pavlovic et al. [20]), P (Kostic et al. [21]) and K (Chen et al. [22]) and metal ion toxicity (cadmium - Shao et al. [23]; manganese - Che et al. [23]; arsenic - Sanglard et al. [24]; aluminum - Wang et al. [25] and Copper - Mateos-Naranjo et al. [26]. As a result of rapid global economic growth, water deficit is becoming an increasing serious environmental problem. At present, the pressure put on agricultural crop cultivation land has caused land degradation, a cultivation shift to large marginal areas and different soil types and qualities, and more requirements for the productivity of agricultural crops per unit area Glick [27]. In addition to undesirable responses on agro-environment ca. 70% of productivity loss in environmental stresses Veatch-Blohm [28].
Low soil water content is one of the common problems constraining crop production in over the world Dinh et al. [29], for ca. 30% of the global fertile agricultural land area. Severe water stress which restricts plant growth, development and photosynthetic characteristics Jangpromma et al. [30]; Graca et al. [31]; Barbosa et al. [32] is responsible for the loss in productivity Ramesh [33]; Zhao et al. [34]. The plants response to water deficit was observed in morphological changes, i.e. plant growth - development InmanBamber et al. [35]; Wilkinson et al. [36], and photosynthesis and plant water potential was also down-regulated. Sugarcane is considered as a renewable feedstock for economically important for sugar and bioenergy production Moore et al. [37-39]. Moreover, the remaining biomass used for the production of second-generation ethanol (2G-bioethanol) Dias et al. [40]; Pereira et al. [41]. The strength of sugarcane to produce high quantity of cane biomass and absorb maximum content of sucrose (ca. 700 mM) in the mature cane stalks Moore [42] are the result of a combination of different factors, including an efficient C4 crop that permits maximum yield of dry matter production hectare-1 Coombs [43].
This experiment investigated the role of Si application on photosynthetic and biochemical activities in sugarcane (cultivar GT 42) under water stress condition. The investigation aimed to understand the impacts of combined experiment with Si and water deficit and to provide evidence demonstrating the biological roles of silicon to tolerate water deficit.
Materials and Methods
Plant Growth and Treatment
This experiment was conducted in the greenhouse of Sugarcane Research Center, Nanning, Guangxi, China. Sugarcane (Saccharum spp. L.) plantlets of cultivar GT 42 was classified in the Sugarcane Research Center of Chinese Academy of Agricultural Sciences (CAAS) and Sugarcane Research Institute of Guangxi Academy of Agricultural Sciences (GXAAS), Nanning, Guangxi, China based on the morpho-physiological behavior and productivity in field experiments/ trials. Before planting, the bud sets were soaked in water for 48 h (Bavistin solution, 1 g L-1 water) for 5 min. Fiftydays after culms germination, plantlets were transferred into the plastic pots (diameter ~30 cm; height ~35 cm with a hole in the bottom) filled with 25 kg air-dried soil (clay soil/ organic manure/ sand, 70:20:10 w/w) with a basal dose of N, P and K fertilizer (N26 g, P-1.76 g and K-20 g pot-1) and kept in greenhouse conditions. Urea (N-46.4%), phosphate (P2O5-12%) and potassium oxide (K,sub>2O-60%) fertilizer were used as a source of N, P and K, respectively. The substrate properties were analyzed before transplanting.
The soil pH was 5.92. Organic carbon (0.72%) was measured through wet oxidation with potassium dichromate Nelson et al. [44]. Available phosphorus was 9.18 mg kg-1, based on the extraction of sodium bicarbonate solution Olsen et al. [45]. Soil available K, Ca, Mg and Na were 2.71, 4.0, 1.6 and 0.083 cmol(+) kg-1 by ammonium acetate method Haby et al. [35]. Soil texture was sandy clay, according to hydrometer methods using 0.5% sodium hexametaphosphate solution Gee [27]. DTPA extractable Cu, Fe, Zn and Mn contents Lindsay et al. [46] were 0.85, 12.0, 1.31 and 18.8 mg kg-1, respectively. Sixty-days after trans-planting, thinning was performed and the three plants per pots were selected for the experiment on the basis of plant morphology. The experiment was arranged in completely randomized block design (CRBD) for the experiment, with four biological replicates of each treatment. All plantlets were irrigated daily upto field capacity to improve root development and growth before initiating treatments. During the experiment, all tillers were removed immediately after emergence.
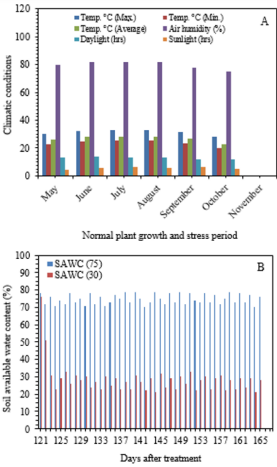
Average soil available water content such as 75 and 30% of soil water was measured by using Soil Moisture Meter (TDZ), Top Instrument Co. Ltd. Zhejiang, China at 15 cm of soil depth. The climatic variables and soil water content (%) were recorded inside the greenhouse during experiment (Figure 1). At 70-days after trans-planting, cultivar GT 42 was submitted to the stress situations (mild -75% and severe - 30% of SAWC) with different levels of silicon application. The average soil available moisture content (%) in treated pots was maintained at 75 ±5 and 30 ±5% during upto the end of the drying cycle (45-days). The experiments consisted of two-groups and six categories, i.e. T0-T5: 0, 20, 40, 60, 80 and 100g Si pot-1 (@25kg soil pot-1). Calcium metasilicate (CaO. SiO2 ), wollastonite powder was used as a source of Si that contains Si-24.18%, O3-41.32% and Ca-34.5%. Si solution was applied two times at an interval of 15 days.
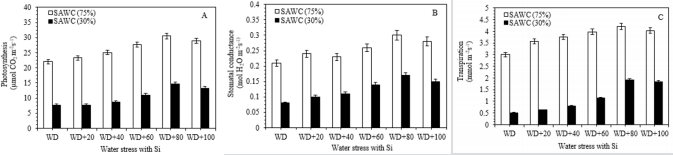
Figure 1: Ameliorating effect of silicon on the (A) photosynthesis (PN), (B) stomatal conductance (gs) and (C) transpiration rate (E) in the top visible dewlap (TVD) leaves of sugarcane cultivar GT 42 under 75 and 30% of SAWC at 45 days after water stress with +Si. Observations were made by Portable LICOR - 6800 System (LI-COR Biosciences, Lincoln, Nebraska, USA). Each value represents the means (±SEM) of at least 3-4 independent biological replicates.
Photosynthetic Measurements
Photosynthesis (PN), stomatal conductance (gs) and transpiration rate (E) were measured in the middle of leaf + 1 (first completely expanded leaf that had a visible dewlap and was photosynthetically mature) using a Portable Photosynthetic System - LI-6800 (LI-COR Biosciences, Lincoln, Nebraska, USA). The CO2 value was fixed at 400 µmol mol-1 by CO2 cylinder, light intensity at 1,000 µmol m-2 s-1 and leaf temperature at 25 ±1 °C.
Quantification of Green Pigments
Chlorophyll a (Chl a), chlorophyll b (Chl b) and total chlorophyll (Chl a+b) were assessed spectrophotometrically according to Strain et al. [47]. Fresh leaves (0.2 g) were removed from the sugarcane plants and extracted with 10 ml of 90% acetone (v/v). Absorbance were measured versus a blank of pure 90% acetone at wavelengths of 663 and 645 nm using an ultraviolet-visible spectrometer for chlorophyll a and b, respectively. Leaf relative water content (RWC- %) was measured according to Yamasaki et al. [48].
Antioxidant Enzymes
Fresh leaves (0.5 g) were frozen in liquid N2 and ground in 10 mL of 100 mM potassium phosphate buffer (pH 7.0) containing 0.1 mM Na2EDTA and 0.1 g of polyvinylpyrrolidone (PVP). The homogenate was then centrifuged at 15,000 * g for 10 min at 4°C. Subsequently, the supernatant was stored at 4°C until being used for catalase, peroxidase and superoxide dismutase enzyme assays. The CAT (EC 1.11.1.6) activity was measured according to Aebi [49]. The decomposition of H2O2 was observed as the decline in absorbance at 240 nm. In this activity, 50 mM phosphate buffer (pH 7.8) and 10 mM H2 O2 were used in the reaction solution. Peroxidase (EC 1.11.1.7) activity was quantified according to Maehly et al. [50] by assessing the oxidation rate of guaiacol in the presence of H2O2 at 470 nm Klapheck et al. [51]. The superoxide dismutase (EC 1.15.1.1) activity was observed based on the inhibition of nitroblue tetrazolium (NBT) photoinhibition according to Giannopolitis et al. [52]. The reaction mixture (3 mL) contained 50 µM NBT, 1.3 µM riboflavin, 13 mM methionine, 75 nM ethylenediamine tetraacetic acid (EDTA), 50 mM phosphate buffer (pH 7.8) and 20 - 50 µL of enzyme extract. The reaction mixture was irradiated under fluorescent light at 75 µM m-2 s-1 for 15 min. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of NBT photoreduction as monitored at 560 nm.
Statistical Analysis
All data shown are the mean values. Data were statistically analyzed by the ANOVA with GraphPad Prism 8.0.1 Software, Inc. Data represented in the tables and figures are means of standard errors (±) of quadruplicates independent replicates of each treatment.
Results and Discussion
Climatic conditions and average soil available water content (%) were observed during the experiment as shown in Figure 1a-b. The air temperature and humidity ranging from 24.5- 30.2°C and 73-80% were higher at 2.05±0.5°C and 7.3±2.0% than those outside of greenhouse, respectively.
Physiological Parameters during Water Stress with Silicon
The photosynthetic characteristics such as photosynthesis (PN), stomatal conductance (gs) and transpiration rate were significantly decreased during 70 and 30% of SAWC. Under the water deficit conditions, +Si showed a higher rate of PN [30.53 vs. 22.01 (~39%) & 14.64 vs. 7.60µmol CO2 m-2s-1 (~15%)], gs [0.30 vs. 0.21 (~43%) & 0.17 vs. 0.08mol H2O2 m-2s-1 (~113%)] and E [4.21 vs. 3.01(~40%) & 1.91 vs. 0.51mmol m-2s-1 (~275%)] at 45 days after treatment, than the –Si plants (Figure 2a-2c). The period and condition of the stress is also most important for plant development under drought stress conditions Passioura [53]. All plants require sufficient amount of essential mineral elements for plant growth and development. Silicon plays positive role in balancing the uptake, transport-distribution of minerals in drought stressed plants Zhu et al. [54,55]; Silicon has been proved to be beneficial for plant growth and development of various plant species Epstein et al. [56-60].
Figure 2: Ameliorating effect of silicon on the (A) photosynthesis (PN), (B) stomatal conductance (gs) and (C) transpiration rate (E) in the top visible dewlap (TVD) leaves of sugarcane cultivar GT 42 under 75 and 30% of SAWC at 45 days after water stress with +Si. Observations were made by Portable LICOR - 6800 System (LI-COR Biosciences, Lincoln, Nebraska, USA). Each value represents the means (±SEM) of at least 3-4 independent biological replicates.
In fact, PN, gs and E of 80-100g Si for 25kg soil pot-1 were significantly higher than those of 20-60g Si, but not significant when plants under stressed conditions without Si fertilizer. Significant differences were found at 45 days after +Si as compare to stress without silicon (Figure 2). Photosynthetic observations are influenced by various environmental conditions, i.e. ambient CO2 level, light intensity, temperature and availability of water Bassi et al. [39]; Sage et al. [61-66] reported that photosynthesis of sugarcane has been related to plant age, and maturity of plants assimilated higher rates than older ones Passioura [67].
Photosynthetic Pigments and Relative Water Content
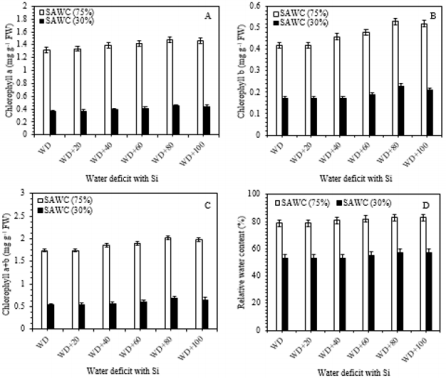
Chlorophyll is considered as an important parameter to verify the level of photosynthetic pigments involved in light absorption and energy transfer during the photochemical process of photosynthesis. Reduction in photosynthetic pigments may mark the start of stress. Results showed in Figure 3, that the decreasing SAWC, i.e. 70 and 30% in the soil was associated with progressive fall in green pigments biosynthesis and leaf relative water content in sugarcane leaves as compared to -Si with stress. The downregulation in chlorophyll may be due to the formation of proteolytic enzymes, i.e. chlorophyllase, which is responsible for pigment declining Sabater et al. [68] as well as damage to the photosynthetic apparatus Yasseen [69]; Kaya et al. [70]. On the other hand, the WD+80g Si pot-1, increased the highest values of Chl a [1.48 vs. 1.32 (12%) & 0.45 vs. 0.36mg g-1FW (25%)], Chl b [0.53 vs. 0.42 (26%) & 0.23 vs. 0.17mg g-1FW (35%)], Chl (a+b) [2.01 vs. 1.74 (~15%) & 0.68 vs. 0.53mg g-1FW (28%)] and RWC [83 vs. 79 (5%) & 57 vs. 53 (~6%)] as compared with 75 and 30% of SAWC without silicon application (Figure 3a-3d).
Figure 3: Chlorophyll a (Chl a; A), chlorophyll b (Chl b; B), total chlorophyll (Chl a+b; C) and relative water content (RWC- %; D) in the leaves of sugarcane cultivar GT 42 under 75 and 30% of SAWC at 45-days after water stress with +Si. The values are the means (±SEM) of four biological replicates.
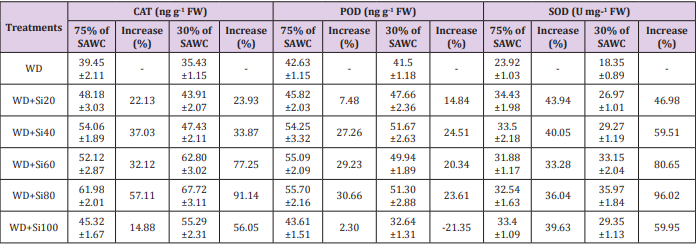
However, there was no further pigment promoting effects from increasing silicon levels (80-100g Si pot-1). Relative water content is known as an alternative method of water level in plants, reflecting the metabolic activities in plant tissues Flower et al. [71]. Similar results were found in many plant species under waterstressed conditions Gadallah [18]; Sairam [70-72]. The reduction in RWC could be due to low availability of water in the soil Kaya et al. [32], which are not able to compensate for loss of water by leaf transpiration through a loss of the absorbing surface Gadallah [73]. Catalase, peroxidase and superoxide dismutase enzyme activities. In comparison with 75 and 30% of SAWC, the activities of catalase [45.32 vs. 39.45 (~15%) & 55.29 vs. 35.43ng g-1FW (56%)], peroxidase [43.61 vs. 42.63 (2.30%) & 41.50 vs. 51.30ng g-1FW (~24%)] and superoxide dismutase [33.40 vs. 23.92 (~40%) & 29.35 vs. 18.35U mg-1FW (~60%)] were significantly increased in stressed plants with +Si. The up-regulated activities of antioxidant enzymes such as catalase, peroxidase and superoxide dismutase in response to drought-stressed plants has been largely used as an indicator of drought avoidance Apel et al. [74-76] reported the enhanced level of CAT in wheat genotypes under water stress was associated with the increased stress tolerance.
Maximum up-regulated enzyme activities such as CAT, POD and SOD were observed in 80g Si pot-1 amended under water stress conditions (Table 1). CAT, POD and SOD were up-regulated under all silicon (20-100g Si pot-1) levels (Table 1). According to Sairam et al. [77], the activities of CAT and POD were highest in severe stress as compare to mild stress in wheat cultivars. CAT, POD and SOD appears to be a more efficient mechanism for maintaining photosynthetic activities in the water stress tolerance varieties. Besides the plant antioxidant defense system against water deficit, silicon can also alleviate oxidative damage in plants Kim et al. [16]. In view of the above findings, Si application to sugarcane plants resulted in maintaining high photosynthesis, stomatal conductance, leaf transpiration, photosynthetic pigments and antioxidant enzyme activities under water deficit conditions [78-81].
Table 1: Specific activity of catalase (CAT, ng g-1 fresh weight), peroxidase (POD, ng g-1 fresh weight) and superoxide dismutase (SOD, U mg-1 fresh weight) in the leaves of sugarcane cultivar GT 42, under 75 and 30% of SAWC at 45-days after water stress with +Si. The values are the means of four biological replicates ± SEM. WD - water deficit.
Acknowledgement
We wish to warmly thank Guangxi Academy of Agricultural Sciences (GXAAS), Nanning, Guangxi, China for providing the necessary facilities for this study. This study was supported in part by the Guangxi Research and Development Program Fund (GK17195100), Fund for Guangxi Innovation Teams of Modern Agriculture Technology (gjnytxgxcxtd-03-01) and Fund of GXAAS (2015YT02).
More BJSTR Articles : https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.