Evaluation of Profile Errors in Drug Infusion by Anesthesiologists: An Overview
Introduction
Patient safety can be defined as the reduction and mitigation of unsafe acts within the health care system, as well as the use of practices aiming at achieving good results for the patient [1]. The culture of patient safety has been progressively becoming a subject of general interest in health [2]. This is not only focused on harmless health care but also on its timely, effective and equitable performance, based on the best scientific information and the full and individual needs of both the patient and the patient. of their family [3]. The national system for reporting adverse events, or potential risk situations, is already known but almost not practiced by health professionals. This system is composed by records and analyzes of the problem origin in the occurrence of patient damage. The aim of these communications is avoid preventable failures, to disseminate this information and introduce changes in the system or practices, in order to prevent the same mistakes from recurring in the future.
The understanding the concept of patient safety is important for problem evaluation and to detect the various factors involved. Studies on diseases caused by health care have been published for some years and the good practices and error decreases resulting from a health care that are crucial to guarantee patient safety in care settings [4]. The goal of these approaches is that the increased and more effective records and analyses of such situations could lead to a reduced occurrence of errors or failures. It has been suggested that the large number of errors observed in medical practice is precisely the absence of mechanisms that diminish its occurrence, or that intercept the error before the patient arrives [5], since it starts from the mistaken assumption that a health professional does not make mistakes and, therefore, no mechanisms of prevention and correction are created. In this way, some related events were defined above.
Adverse Reaction
The adverse drug reaction is defined by the World Health Organization (WHO) as any unintentional and unintended harmful event occurring during the use of a medicinal product used at usual therapeutic doses for treatment, prophylaxis or diagnosis.
Adverse Events
The adverse event is defined as the occurrence, in humans, of any undesirable effect resulting from the use of products under sanitary surveillance. This has to be reported to the responsible sectors of health institutions and / or regulatory agencies. Promoting the adoption of preventive measures ensures greater patient care. (Resolution-DRC No 23 from Apr. 4th, 2012). The adverse drug event involves different situations, including adverse drug reaction and therapeutic ineffectiveness [6]. According to TOMÁS and GIMENA [7], the incidence of adverse events in emergency services is estimated between 1.6 and 14%, considering different studies and methodologies adopted. In Brazil, a study with patients admitted to hospitals in Rio de Janeiro observed 5.6% of adverse drug events [8]. Another study carried out in the country, in the Center-West region, revealed a frequency of 8% of adverse drug events in hospitalized patients [9] and the adverse events to severe drugs are the most reported because they cause harm to patients [10]. Thus, the importance of monitoring and reporting of adverse events is evidenced, justifying the accomplishment of this communication.
Medication Error
Medication error, according to the National Coordinating Council for Medication Error Reporting and Prevention is any avoidable event that causes or induces the inappropriate use of a medication, being the medication in the control of the health professional, patient or consumer [11,12]. Researchers who studied the subject in Brazilian public hospitals, have identified problems in the administration of 30% of cases4, in the present communication, it was observed that the use of drug in the treatment of disease is not a problem. National Agency of Sanitary Surveillance [13] states that medication error is any avoidable event that, in fact or potentially, may lead to inappropriate use of medication. The error can be related to professional practice, products used in the area of health, procedures, communication problems, including prescription, labels, packaging, names, preparation, dispensing, distribution, administration, education, monitoring and use of medicines. It is important for institutions to encourage notification and for professionals included in the practice to have awareness of their importance beyond their knowledge. The lack of notification of the error related to medication is a worldwide problem, because even today there is a punitive culture, compromising the veracity of the data [14].
Causes of Medication Errors
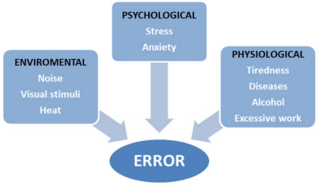
The commitment with medicine is from different health professionals, such as doctor, by prescription; pharmacist, for the release and distribution; and the nursing team, by management and supervision. It is crucial to each step of the medication process, however, in the preparation stage, the error must be distinguished, since it is the stage that precedes administration to the patient [15]. Non-existent knowledge and inexperience were also identified as relevant factors for the occurrence of errors [3]. Yamamoto [14] also reported that the higher incidence of errors with medication was associated with the incorrect rate of drug infusion. It was then realized that this type of error is linked to the infusion pump programming and its malfunction due to the handling, proving that the training of the professionals is of paramount importance. Most of the errors observed in medical practice were noted by the absence of mechanisms that diminish their occurrence, or that intercept the error before reaching the final consumer - the patient. According to Carvalho et al. [15] are environmental, psychological and physiological factors combined that provide the error in the practice of medicine. The figure below presents the main factors that interfere in the occurrence of errors (Figure 1).
ANVISA Legislation
According to the National Agency for Sanitary Surveillance (ANVISA) Medicines Labeling Regulation16, parenteral solutions, families of similar medicines should be of the same label color, this color being pre-established in a specific annex. It is, however, clear that medications with different potencies and similar names, such as Ephedrine and Epinephrine (Figure 2), may be confounding factors. It can still be mentioned the fact that there is nothing on the form of ampoules, but drugs of quite different potencies, but from the same family have the same color of the label and the same ampoule format, these factors being confusing, especially in situations of urgency (Figure 3).
Conducting the Medication Error
Describing failings and explaining their cause and reason for that, in health area or elsewhere, is not only to talk about people-related issues, but also analyze and detect the external circumstances and environmental factors that provide this human error [12]. Among the activities with the purpose of promoting scientific knowledge, it is worth mentioning continuing education, an approach that allows the development and improvement of health professionals and ensures the quality of customer service [16,17].
To aim these procedures ANVISA, also recommends a medication error prevention program based on the following topics:
a) Promotion of search and identification of human and institutional errors.
b) Promoting the prevention of accidents in health care.
c) Encouraging the incorporation of new knowledge on the origin of medication and patient safety.
d) Raising awareness and creating communication to improve patient safety.
e) Development of information, collaborative and educational approaches that promote patient safety.
Many studies present continuing education as a way to minimize avoidable medical failures. From these findings, it becomes important to evaluate and study possible medical errors that can cause damage to the patient´s health.





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.