Analysis of Oxidative Stress Markers in Plasma Derived from Individuals Exposed to Ionizing Radiation
Introduction
Ionizing radiation with low-linear energy transfer generates reactive oxygen species (ROS) or free radicals such as superoxide, hydrogen peroxide, and hydroxyl radicals by indirect action of energy on water molecules and simultaneously generating doublestrand breaks (DSBs), which are known to be lethal, by direct action on DNA itself, with sequence apoptosis or stress-related responses [1,2]. Redox imbalance, caused by a decrease in antioxidant activity and a rise in ROS generation, can elicit an increase in oxidative molecular damage, such as lipid peroxidation, protein denaturation, or DNA mutations [3], following the induction of oxidative stress. As a result, an abnormal ROS accumulation has been implicated in the pathogenesis of various diseases, including cancer, atherosclerosis, diabetes mellitus, and neurodegenerative disorders [4].
Reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) are used to evaluate the antioxidant activity and degree of oxidation in organisms. The level of hydroperoxide, which is a chemical oxidant species belonging to the active oxygen metabolites, can be measured using the d-ROMs test. Similarly, various levels of antioxidants such, as ascorbic acid, uric acid and bilirubin, can be measured using the BAP test [5]. These tests are known for the rapidity, simplicity and high reproducibility of their methodologies. In addition, the d-ROMs test can conveniently estimate diseases such as hypertension, hyperlipidemia, diabetes and chronic obstructive pulmonary disease [5,6]. Although oxidative stress markers in the plasma of individuals subjected to radiotherapy can be assessed with the d-ROMs test [6, 7], no studies have yet evaluated both the d-ROMs and the BAP tests. Since it is important to determine the oxidative conditions of individuals exposed to ionizing radiation, the aim of the present study was to use the d-ROMs and BAP tests to assess oxidative stress in irradiated mice.
Materials and Methods
Mice
Female C57BL/6 mice were delivered at 7 weeks of age from the breeding facilities of Clea Japan, Inc. (Tokyo, Japan) and housed in a conventional animal room with a 12h light/dark cycle. The mice received food and water ad libitum. At 8 weeks of age, the mice were exposed to 0.5, 2 or 7 Gy total body irradiation at a dose rate of 1.0 Gy/min and settings of 150 kV and 20 mA using a Hitachi MBR1520R-3 X-ray irradiation device (Hitachi, Ltd., Tokyo, Japan). The beam was filtered through 0.5mm aluminum and 0.3mm copper plates. All experiments were conducted according to the legal regulations in Japan and the guidelines for Animal Experiments of the Hirosaki University after obtaining approval from the Animal Experimental Committees of Hirosaki University.
Isolation of Bone Marrow Mononuclear Cells (BM-MNCs)
The femurs of the mice were harvested and the BM-MNCs were collected in 0.5% bovine serum albumin/EDTA/calciummagnesium-free phosphate-buffered saline. Bürker–Türk solution (Nacalai Tesque, Kyoto, Japan) was used to count the collected BMMNCs.
Measurement of Free Radical Metabolites in the plasma
Peripheral blood was collected from the mice under isoflurane anesthesia via retro-orbital venous plexus puncture into heparinized hematocrit tubes. The plasma was isolated by centrifuging the collected peripheral blood at 100 g for 10 min. A d-ROMs test U-kit (Wismarll Co., Ltd., Tokyo, Japan) was used for the measurement of the free radical metabolites in the plasma samples [8]. This test comprises a spectrophotometric method that evaluates the overall oxidative stress by measuring the total hydroperoxide levels, since hydroperoxides are the intermediate oxidative products of peptides, amino acids and lipids [9].
Upon diluting the plasma in an acetate-buffered solution, the hydroperoxide groups react with the transition metal ions liberated from the proteins by the acidic medium and are converted to alkoxyl and peroxyl radicals according to the Fenton reaction [10]. These newly formed radicals are then chemically trapped using a chromogen [N, N-diethyl-p-phenylendiamine (DMPD)], leading to the formation of the radical cation of this chromogen and yielding a purple color. This colorimetric reaction was monitored by a spectrophotometer (FRAS4; Wismarll Co., Ltd.) at 505 nm. The results of this method were expressed in the conventional Carratelli units [6].
Assay of Antioxidant Activity
A BAP test kit (Wismarll Co., Ltd.) was used to measure the antioxidant activity [9]. Plasma was dissolved in a colored solution that was previously prepared by mixing ferric chloride with a thiocyanate derivative. Upon plasma dissolution, the ferric ions in the solution were reduced to ferrous ions through the plasma antioxidant activity, causing the solution to lose its color during incubation (37˚C, 5 min). This change in absorbance was measured using the FRAS4 spectrophotometer.
Statistical Analysis
The statistical analysis was performed using the Excel 2013 software program (Microsoft Corp., Redmond, WA, USA) with the Statcel 3 add-in software package [11]. The Tukey-Kramer test was used to analyze the data. P<0.05 was considered to indicate a statistically significant difference.
Results and Discussion
Number of BM-MNCs
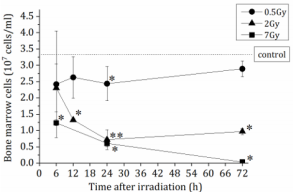
The mice were exposed to 0.5, 2 or 7 Gy X-irradiation, following which the BM-MNCs were counted at 6, 12, 24 and 72 h postirradiation (Figure 1). In the group exposed to 7 Gy irradiation, the number of BM-MNCs at 6h post-irradiation was significantly decreased to ~40% of the number in the non-irradiated controls and ultimately decreased to <1% of the number in the non-irradiated group. A similar trend was observed in the group exposed to 2 Gy irradiation; however, no significant decrease was observed in the number of BM-MNCs in the mice exposed to 0.5 Gy irradiation.
Figure 1: The total number of bone marrow mononuclear cells (BM-MNCs) in femurs of non-irradiated control mice (n=9) or X-irradiated mice (n=3-6). The number of BM-MNCs were counted using Burker-Tulk solution. The data are expressed as the means ± SD. *P < 0.05 and **P < 0.01 versus non-irradiated control.
BAP and d-ROMs Analyses of Murine Plasma
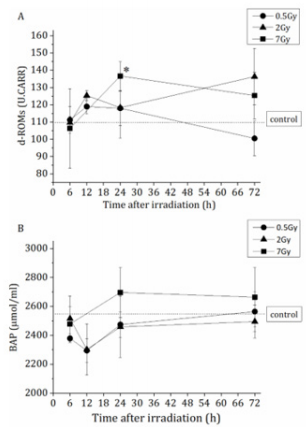
The levels of oxidative stress generated in murine plasma were evaluated by measuring the free radical metabolite and antioxidant activity levels. Murine plasma was collected from the peripheral blood of each treated individual and then subjected to BAP and d-ROMs testing. The d-ROMs values, which indicate the levels of free radical metabolites, were significantly increased at 24h post-irradiation with 7 Gy (Figure 2A); however, no significant differences were observed between either of the other irradiation groups and the non-irradiated control group at any time-points. In comparison, the BAP values, which indicate antioxidant activity, reached their lowest points at 12 h post-irradiation with 0.5 and 2 Gy and then returned to their initial levels. No significant differences from the control results were observed at any time-point (Figure 2B). The method described by Verde et al. [12, 13] was used for the analysis of the derivatives of the d-ROMs in the plasma. The d-ROMs test measures oxidative stress in blood samples by determining the levels of d-ROMs, particularly hydroperoxides.
Figure 2: Oxidative stress marker levels in plasma derived from non-irradiated control mice (n=6) or X-irradiated mice (n=3- 6). (A) The values of d-ROMs which shows free radical metabolites. The data are expressed as the means ± SD. *P<0.05 versus non-irradiated control. (B) The values of BAP which shows antioxidant activity. The data are expressed as the means ± SD. Statistical significance was determined by the comparison of the irradiated group with the non-irradiated control group.
The BAP assay is based on the ability of DMPD to yield a stable, colored solution following its conversion into a radical cation (DMPD+) [14]. This assay is a photometric test that determines the serum antioxidant concentrations capable of reducing ferric iron to ferrous iron [14]. It has been demonstrated in several studies that the d-ROMs test is a useful oxidative stress marker that may be used to predict the presence of several diseases [13,15,16]; however, no previous studies have used the BAP/d-ROMs tests to evaluate the plasma of individuals that have been exposed to ionizing irradiation. The present results suggest that d-ROMs levels, but not the BAP index, could be used as a prognostic indicator of radiation exposure. In the previous study, it is reported that ionizing radiation induces secondary ROS production from mitochondria in human lung carcinoma A549 cells, which is accompanied by elevated mitochondrial contents and upregulation of the mitochondrial electron transport chain function [17]. Secondary ROS production may be considered to have increased d-ROMs values. Additional studies aiming to examine the mechanism underlying this association between d-ROMs and radiation exposure are currently being conducted in our laboratory.
Conclusion
d-ROMs measurement could serve, to a certain extent, as a prognostic indicator of radiation exposure.
Acknowledgment
This work was supported in part by KAKENHI, a Grant-in-Aid for Scientific Research (No. 25293256 IK).
Conflicts of Interest
The authors report no conflicts of interest.
More BJSTR Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.