Potassium Channel and Glioma
Introduction
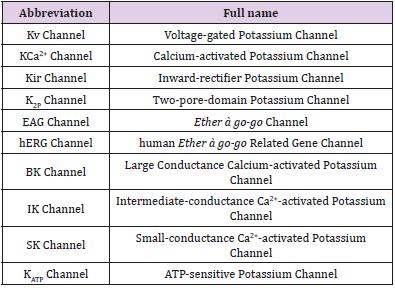
Ion channels have been shown to play a pivotal role in the origin of various cancers in the literature. They become a very useful and accessible target for modulation. Among those under study, potassium channels are the best characterized with established role in cell cycle regulation. Potassium channels are transmembrane proteins that are defined by their ability to selectively facilitate the permeation of K+between intracellular and extracellular environments [1]. Under resting conditions, nearly all of the ions that move across the membrane (intracellular to extracellular) are K+ions, and these results in a negative membrane potential [1]. According to conductance properties, structural criteria, and whether combine with stimulus, potassium channels are divided into four classes: Kv channels, KCa2+channels, Kir channels and K2P channels. Potassium channels are one of the most widely distributed ion channels and play an important role in the development of many diseases [2]. Recent studies found many potassium channel subtypes abnormally expressed and regulated cell biological behaviors, such as the leiomyosarcoma aggressiveness [5,6].
Therefore, some scholars have classified the malignant tumor into the “Potassium Channel Disease” category [7-9] and regarded potassium channels as the promising therapeutic target. Accumulating evidence has proved that a variety of potassium channels, including Kv channel [10-12], KCa2+channel [13], K2P channel [14] and Kir channel [15] are overexpressed in tumorous tissues compared with their healthy counterparts. Consistently, it was found that the potassium channels, which were significantly unusual expression in glioma, were mainly Kv channels (such as EAG1[16], hERG [17]), KCa2+channels (BK [18-20], IK [18,21], KCa3.1 [22] and SK[18]) and KATP channels (such as Kir6.2 [15,23]), and they were highly correlated with the malignancy of gliomas. Potassium channel blockers such as 4-AP (4-aminopyridine, Kv blocker), tolbutamide (KATP channel blocker) could significantly influences the growth of glioma [15,24]. Therefore, potassium channels play an important role in the development of gliomas.
Expressions of Potassium Channels and Gliomas
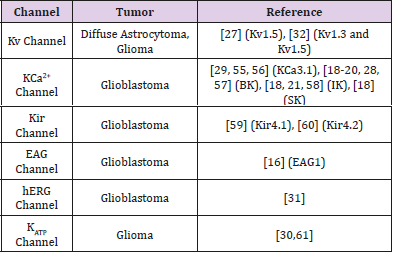
K+ channels selectively expressed in multiple types of tumor cells, and deeply influenced on biological behavior of tumors, such as proliferation, apoptosis, differentiations and invasions. However K+ channels didn’t expressed or down-expressed in normal tissues, such as mammary glands [24], prostate glands [25, 26]. This selective expression in tumor tissue indicates potassium channel may be the potential therapeutic target and has important clinical application value. Gliomas are the most common malignant brain tumors, and numerous studies have shown that there are many kinds of potassium channels expressed in glioma cells, such as Kv channels [27], BK channel [28], KCa2+ channels [29], KATP channels [30] and hERG channels [31] (Tables 1& 2). Preussat [32] first found the levels of expression of Kv1.3 and Kv1.5 subtypes discriminated between various glioma groups, and a clear differential expression of Kv1.5 was observed according to malignancy grade. Later, Debska-Vielhaber [28] found that LN229 cell (human glioma cell line) expressed BK channel. What’s more, Basrai reported that BK channel was found in the human glioma cell line STrG-1 (anaplastic astrocytoma, WHO grade III) and D54-MG (glioblastic glioblastoma, WHO grade IV).
EAG channel has been shown to express in numerous tumor tissues, and may closely associate with tumor generation, malignant growth, invasion and metastasis[31,33-35]. hEAG channels belong to the family of Kv channels with delayed rectifier characteristics [31]. Patt [35] found a differential expression of hEAG1 and hERG1 in gliomas depending on the malignancy grade and nature of the tumor cells. Catacuzzeno [36] demonstrated that K+ ion flux was essential for the FCS-induced glioblastoma cell (U87-MG) migration. Not only in the human glioma cell line , but also in the animal glioma. Zhu [37] found that Kv1.3 and Kv1.6 channel could be observed in rat astrocytes. And recently, Venturini [38] report that Kv1.3 is expressed in mitochondria of human and murine GL261, A172 and LN308 glioma cells. Treatment with the novel Kv1.3 inhibitors induced massive cell death in glioma cells. These studies have supported that potassium channel may involve in the process of gliomas.
Potassium Channels and Cell Cycle of Gliomas
The cell cycle is divided into defined phases, namely G1 (first gap), S (synthesis), G2 (second gap) and M (mitosis), while a post-mitotic cell in G0 is considered to be in a non-dividing status (quiescent). While cancer cells generally maintain moderately depolarized membrane potential compared with nontransformed cells, transient hyperpolarization has been reported to be necessary for successful G1/S cell cycle progression [8,33]. In spinal cord astrocytes down-regulation of Kir accompanied with a depolarization was observed to promote cell cycle progression through the G1/S checkpoint. This indicates depolarization to be necessary for entering the S phase [39]. Using medicines blocking the Kv channels and KATP channels in U87-MG could inhibit the growth of tumor via an arrest in the G0/G1 transition during the cell cycle [30]. Samely, Huang [15] reported that treating U251(glioma cell line) cells with the blocker of KATP channels blocked cell cycle in G0/G1 phase, while a block of delayed rectifier potassium channel caused proliferating astrocytes to arrest in G0/ G1. What’s more, Klumpp [40] recently reported that blocking the intermediate-conductance Ca2+-activated K+channel KCa3.1 could force G2/M cell cycle progression in GL261 glioma cells treated with the DNA-alkylating drug temozolomide, and then facilitates apoptotic cell death.
In summary, there are evidence suggesting that several different types of potassium channel-ligand-as well as voltagegated or combinations of these channels are necessary for cells to progress through the cell cycle. The reason for this effect was attributed to K+diffusion through potassium channels out of the cells as shown in theoretical models resulting in hyperpolarization of the membrane potential [7,41].
Potassium Channel and Proliferation/Apoptosis of Glioma
Given the important role of potassium channels in tumors, it is necessary to clarify their role in proliferation. Accumulating evidence has indicated that potassium channels are relevant players in controlling cell proliferation and apoptosis of various tumor cells, and pharmacological blockades of Kv channels lead to cell proliferation inhibition [42]. Electrophysiological and pharmacological results proved that quinidine inhibited cell (U87- MG cell [43] and C6 glioma cell [44]) proliferation and apoptosis in the concentration range required to block Kv channel currents. This indicates that quinidine potentially inhibited cell proliferation and induced apoptosis by blocking Kv channel activities. Since KATP was found also involved in regulating numerous cellular functions. Ru [30] proved that Kv and KATP channel blockers inhibited proliferation and tumorigenesis of U87-MG glioma cells. It was likely that potassium channels activities modulated Ca2+influx into U87-MG cells and therefore affected the proliferation and apoptosis. Huang [15] studied the effect of KATP channels activity on glioma cells proliferation, which is mediated by ERK (extracellular signalregulated kinase) activation.
They found that activation KATP channel triggered ERK activation and inhibiting KATP channel depressed ERK activation. Abdullaev [13] proved that downregulation BK channels in U251 cells using gene-specific siRNA didn’t affect the rate of proliferation, while paxilline (inhibitor of BK channel) reduced both U251 and U87-MG cells proliferation in an additive fashion. Hao [45] down-regulated TASK-1 by the transfection of siRNA improved the proliferation rates of N2A cells, suggested that this channel was involved in the regulation of neuronal growth. What’s more, Staudacher [31] found that suppression of hERG protein is a crucial molecular event in glioblastoma cell (LNT229 and U87-MG) apoptosis. Recent study [43] has shown that Kv channels are expressed in the inner mitochondrial membrane. Kv3.4 inhibition blocked MPP+(1- Methyl-4-phenylpyridinium ion)-induced cytochrome c release from the mitochondrial intermembrane space to the cytosol and mitochondrial membrane potential depolarization, which are characteristic features of apoptosis [46,47]. The finding of Szabo study [48] demonstrated that mitochondrial Kv1.3 channel mediated Bax-induced (Bcl-2 associated protein X) apoptosis, and Cheng [49] found that mitochondrial potassium channels play a central role in the induction of apoptosis by Bax. Similar to these, Ru [50] reported that blocking the Kv channels would induces glioma cell apoptosis by reducing expression of microRNA-10b-5p.
However, some studies hold the different opinion. Debska- Vielhaber [28] showed BK channel openers CGS7181 and CGS7184 induced glioma cell death, and this effect was due to the modulation of calcium homeostasis by BK channel openers leading to activation of calpains. Li [51] found that the blockage of Kv channels could improve the proliferation of N2A cells. Potassium channels may use several mechanisms to regulate cell proliferation. The induction of tumor growth via the abnormal expression of potassium channel subtypes since Ca2+ acts as an activator involved in many cellular signal transduction pathways, including the cell growth and mitosis pathways [52]. Hyperpolarization increases the driving force for Ca2+ into the cells according to the Nernst equation, which makes sense since Ca2+ is another major factor in cell-promoting proliferation. Ca2+-sensitive potassium channels like BK channels may serve as regulatory sensors by hyperpolarizing cells and in this way limit the action of voltage-operated Ca2+ channels.
And in another hand, the cell membrane of the tumor cell is more polarized than the normal cell, and the early stage of G1 phase in cell cycle requires a brief hyperpolarization, while blocking potassium channels results in depolarization which ceases cell proliferation.The studies that whether potassium channel blockers promote tumor cells’ apoptosis are rarely published. But some believe that the activation of potassium channel and the outflows of potassium and Cl- are necessary to change cell volume before apoptosis, thus blocking potassium channels cause the inhibition of apoptosis [41].
Blockers of Potassium Channel and Treatments of Gliomas
The multiple drug resistance (MDR) of tumor cell associated with a variety of mechanisms is a significant obstacle in tumor therapy. However, the main form of resistance related to membrane glycoprotein. Glycoproteins pump the toxic substances out of cells and make the intracellular drug concentration lower than the threshold of killing cells. In MDR phenotypic cancer cells, high expression of membrane glycoproteins is associated with increased intracellular K+current. It has been found that a large number of toxins and drugs have the ability to regulate potassium channels, which can be used to block potassium channels or change channels’ sensitivities to voltage and calcium concentration.The development of the tumor requires the acceleration of proliferation and the weakening of apoptosis. The inhibition of potassium channel blockers to cell proliferation is related to cell volume, transmembrane potential, and cell cycle. Yang [53] proved blocking potassium channel using TEA (tetraethylammonium) could inhibit rat glioma cell lines (C6 and 9L) proliferation and induce apoptosis in both cell lines, and it might be associated with the increase in intracellular ROS (reactive oxygen species) production.
Ru [43] found that quinidine (a commonly used Kv channel blocker) significantly inhibited the proliferation of U87-MG cells and induced apoptosis in a dose-dependent manner. Sales [34] proved that silencing EAG1 is a promising strategy to improve glioma treatment. Newly, there is a compounds , senicapoc which is made with KCa3.1 blocking tool, has previously been in Phase III clinical trials. And this medicine can cross the blood brain barri er, which means it would be available for repurposing, and could be used to quickly translate findings compounds into clinical trials [54]. The opening of potassium channels can release the non-voltage sensitive calcium and activate the Ca2+ channel, which makes Ca2+ enter intracellular space and participates in the Ca2+ related signaling pathway, then accelerate tumor cells’ proliferation. Potassium channel blockers can inhibit this process, therefore inhibit the tumor cells’ growth. Blockage of potassium channels not only can inhibit the growth of tumor cells, but also can induce the apoptosis of tumor cells. Therefore, we could view this as a potential therapeutic target in cancer treatments [55-57]. In the early stage of tumor cell generation, potassium channel blockers were used to prevent excessive proliferation, while the tumor cells were killed by potassium channel activators in the stage of terminal cancers. Therefore, inhibition of glioma cells by potassium channel blockers and its specific mechanism remains to be further studied.
Summary
The expression of K+channels of different subtypes has been confirmed in various glioma cells or tissues, and the blockade of K+channels often affects a variety of cellular activities. In addition, the potassium channel can also as the gene therapy target of cancer treatment. Such as using knockout, antisense oligo nucleotide can inhibit the growth of tumor. The role of K+channel inhibitors in inhibiting glioma cell growth can provide new insights into the treatment of glioma. However, the cellular mechanism regulated by K+channels is extremely extensive and the role of K+channel blockade at the level of glioma tissue is still lacking in a large amount of experimental data. Therefore, further study of the role of K+channels in the development of gliomas and verify its effects at the tissue or even the individual, is necessary for the development of K+channel targeted drugs for glioma.
Acknowledgement
This project was supported by the National Natural Science Foundation of China (No. 30970353), and the Science and Technology Plan Projects in Liaoning Province, China (No. 2015020568).
More BJSTR Articles: https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.