Study of Dimer Lead Compounds via Triazole Rings as Novel Potential Antitumor Agents
Introduction
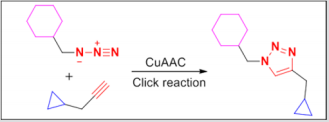
Cancers still remain the second leading cause of death worldwide after cardiocascular diseases. It is estimated that about 18.1 million new cancer cases and 9.6 million cancer deaths in 2018 [1]. However, the medicines for treatment of malignant tumors are far from sufficient. So the development of novel agents with good therapeutic is the key topics which have been concerned currently [2]. Natural products based drug discovery has become a major strategy in modern pharmaceutical research [3], and roughly 60% of the currently used cancer chemotherapeutic agents are directly or indirectly derived from natural products, but the high toxicity, the lack of “true” tumor cell specificity, and the narrow therapeutic margins of the most natural compounds has limited their application as a drug in cancer chemotheraphy [4-7]. Recently, more attention has been attracted on targeting anti-cancer drugs: disrupting tumor-specific cell signaling, cell division, energy metabolism, gene expression, drug resistance and so on. The structural modification of natural compounds for developing new antitumor drugs with increased selectivity and reduced toxicity is highly desirable. For example, the 1,2,3-triazole ring is a widespread functional group in various drugs [8]. The Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction (Click reaction) was a powerful means for linking two molecular fragments between a terminal alkyne and an azide to generate substituted 1,2,3-triazole (Figure 1) [9,10]. Click reaction has recently emerged as one of the most powerful tools in drug discovery, chemical biology, and proteomic applications.
Figure 1: The Cu(I)-catalyzed Husigen azide-alkyne 1,3-dipolar cycloaddition reaction (Click reaction).
A dual inhibitor is likely to reduce the liabilities associated with combination treatments, particularly, off-target toxicities, drugdrug interactions, and additive effects [11]. In addition, dual target inhibitors would likely retain cytotoxic activity when resistance was acquired due to alteration of only one drug target. Thus, dual inhibitors are an attractive therapeutic approach in the drug development process. The Click reaction can link two molecular via 1,2,3-triazole ring, the strategy of dimer lead compounds via triazole rings as potential antitumor agents shown in Figure 2. As illustrated in Figure 2, two drugs (same or different drugs) linked via a triazole ring with the different length of the linking spacer between the drug and the 1,2,3-triazole (Figure 2I); or two drugs (same or different drugs) linked via two triazole rings with the different length of the linking spacer between the drug (1,2,3-triazole ring) and the 1,2,3-triazole (Figure 2 II).
Recently, the Click reaction has been gaining popularity link two molecular fragment in creating a wide variety of drug-like molecules [8,12], such as paclitaxel [13], podophyllotoxin [14,15], and camptothecin [15]. Unpublished findings from our laboratory suggest that dimeric epigallocatechin gallate/ camptothecindervatives, and epigallocatechin gallate-4β-triazolopodophyllotoxin/camptothecin conjugates showed highly potent anticancer activity. Thus, the strategy of dimer natural active lead compounds via triazole rings can promote the development of double target inhibitors which reduces the length and complexity of trials as well as costs.
Conclusion
Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction was employed successfully to prepare a series triazole-linked derivatives. The available data so far produced suggest that the dimer lead compounds via triazole rings might be an innovative strategy for potential antitumor agents. It is extremely important to continue the discussion about the dimer lead compounds via triazole rings for development of cancer chemotherapeutic agents in further studies.
Acknowledgment
This work was supported by the National Nature Science Foundation of China for financial support (No. 21602196); the Yunnan Provincial Science and Technology Department (Nos. 2017ZF003-04, 2017FD084 and 2017FG001-046); and Yunnan Agricultural University Natural Science Foundation for Young Scientists (No. 2015ZR08).
For more Articles: https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.