Effect of Sugarcane Smut (Ustilago scitaminea Syd.) on Ultrastructure and Biochemical Indices of Sugarcane
Introduction
Sugarcane smut, caused by Ustilago scitaminea Syd., is one of the most severe fungal diseases affecting sugarcane productivity, and all producing countries have developed protocols for the protection and control of this disease [1,2], also a significant decrease in sucrose content. Its sucrose accounts for ca. 80% of the total sugar production globally, and about 90% of the total sugar production in China. China reported that the disease occurred in Guangzhou in 1932. Diseases are becoming more serious in sugarcane main producing areas, i.e. Guangxi, Guangdong and Yunnan [3]. Smut disease caused loss of sugarcane yield in upcoming years, especially in dryland and perennial sugarcane [4-6], also known as “the death of sugar cane” [3]. One of the most obvious features of this disease is that the diseased cane has a curled black whip that varies from a few to tens of centimeters [7]. The disease can be transmitted in a variety of ways, especially its teliospores can spread over a wide range and long distances with the wind [8].
In addition, the transportation of contaminated sugarcane has become the main method of long-distance transmission [3]. Many studies have shown that the younger inoculation of the upper part of the cane bud is more susceptible than the inoculation of the base bud, that is the young shoot is more susceptible than the old shoot [9]. The invention of electron microscopy has further developed cytological studies that allow plants to interact with pathogens. The cellular changes caused by the interaction of different host plants with different pathogens are also different. [10] studied the interaction between rice and rice blast fungus, and found that the arrangement of microtubules and microfilaments in the sheath of rice was very intense after infestation by rice blast. The cell wall is intact, but the cytoplasm is highly aggregated or the organelles are disintegrated [11]. Wenying et al. [12] found that under the stress of sweet potato scab, the ultrastructure of leaf cells of different disease-resistant sweet potato varieties changed significantly, the cell membrane was destroyed, and the chloroplast and mitochondria were also damaged to varying degrees.
The predecessors studied the relationship between various physiological and biochemical metabolic reactions of host cells and plant disease resistance after infecting plants with pathogens. The SOD and POD activity can resist the damage of active oxygen and oxygen free radicals on the cell membrane system, while PAL and PPO can promote the production of a variety of secondary metabolites in plants, thereby preventing the invasion and reproduction of pathogenic bacteria [13-16]. Liping et al. [17] suggested that peroxidase had a certain correlation with sugarcane resistance to smut. The more susceptible varieties were infected with smut, the enzymatic activity increased relatively. The enzymes that act as cell wall hydrolase in defense responses against pathogen infection are β-1,3-glucanase and chitinase [18]. Plant endogenous hormones are regulators of plant life activities, which are related to the main physiological and biochemical reactions during plant growth, which in turn affect plant growth [19]. Sugarcane disease is the main biological stress of sugarcane production.
After smut infection, the cells in the body will undergo a series of adaptive changes in morphological, physiological and biochemical to protect against pathogen infection. The morphological structure of plants is always compatible with the environment. Adversity affects plant growth and can cause corresponding changes in plant morphological structure. The ultrastructure of host cells will change significantly due to the infection of pathogenic bacteria [20]. During the invasion from the pathogenic fungus to the manifestation of the disease, the plant has undergone a series of morphological changes, including external morphology and internal structure. The external feature of sugarcane plants is a visual representation of the appearance of sugar cane. The physiological metabolism process of plants is related to the structure of cells, and the interdependence between them is very close.
Materials and Methods
Plant Material and Inoculation
The selected plant material was sugarcane variety ROC22. The pathogen for inoculation was collected from the sugarcane base field of Guangxi University Agricultural College, Nanning, Guangxi, China. After drying, it was sealed with a plastic bag and stored in a -80°C. The smut pathogen was inoculated by artificial dipping method, and the spore germination rate of the smut disease was over 91%. A suspension of smut germs at a concentration of 5 x 106 spores/ml was prepared with 25 g of spores and 10 kg of water [21]. 100 healthy single buds were taken for the test materials. After the warm soup was disinfected, the average was divided into 2 parts, one of which was immersed in the prepared spore suspension, taken out after 30 min, moisturized with a plastic bag and placed at ca. 25°C. The barrel was planted 24 hr after the temperature in the greenhouse. The blank control replaces the bacterial spore suspension with sterile water, and the rest of the operation is the same.
Preparation and Observation of Ultrastructure
Cut the healthy and susceptible sugarcane buds with a doublesided blade. The strips cut to a size of 1 × 2 cm were placed in a 2 cm diameter vial for aspiration treatment. After evacuation, the material was removed and surrounded by a fixative. Edge excision, finally cut the material into l × 2 mm tissue pieces, immediately put the material into 4% paraformaldehyde and 2.5% glutaraldehyde (0.1 mol / L phosphate buffer, pH 7.2) 4°C After fixing for 24 h, 0.1 mol/L phosphate buffer (pH 7.2), 1% citric acid (0.1 mol/L phosphate buffer, pH 7.2) was fixed at 4°C overnight, and 0.1 mol/L (pH 7.2). After washing with phosphate buffer, it was dehydrated with a series of alcohol gradients, then transferred with acetone. Spurt resin was infiltrated and embedde and sliced on an ultrathin slicer. After staining with uranyl acetate and lead citrate, respectively. To observations were made by transmission electron microscope (TEM).
Enzyme extraction
The sample +1 leaf and cut off the two ends about 3 cm, remove the midrib, weigh 1 g of the sample, quickly cut it, put it into the mortar precooled with liquid nitrogen, add a little quartz sand and 0.1 g PVP, add liquid nitrogen grinding crush, add 2 mL of extract and quickly grind into homogenate. Transfer the homogenate into a 15 ml centrifuge tube, then take 2 mL of the extract and collect the homogenate on the mortar and pestle. At 4°C, the tube was shaken for 5 min, placed in an ice box, centrifuged at 12000 r/min for 4 min (4°C), and the supernatant was taken. The supernatant used for biochemical enzyme assays.
Determination of POD, SOD, PPO, Chitinase and β-1,3- glucanase indices
According to Baoju and Fengyun, 1998, slightly modified. Take 0.1 mol/L (pH 5.8) phosphate buffer (containing 18 mmol/L guaiacol) 4.8 mL into a 10 mL tube and mix well with 50 μL of the crude enzyme extract. Then, it was placed in a 35°C water bath for 1 min, and measured wavelength of 470nm. Slightly modified to the method of Chen et al. [22]. The enzyme reaction, i.e. 4 mL of mixed reaction solution containing 1.3 μmol/L riboflavin, 13 mmol/L methionine, 63 μmol/L NBT, 0.05 mol/L phosphate buffer (pH 7.8). The enzyme activity was measured 570nm wavelength, expressed by inhibiting 50% of the NBT photochemical reaction as an enzyme activity. Take 0.1 mol/L pH 6.8 phosphate buffer (containing 0.02 mol/L catechol) 4.9 mL into a 10 mL tube, and add 100 μL of the crude enzyme extract. After reacting for 5 min in a constant temperature water bath at 35°C, the absorbance was measured at 398nm by colorimetry method. The extraction of chitinase solution was carried out according to the method of Boller et al. [23]. The chitinase activity was determined by Tang [24]. The activity of β-1,3-glucanase was determined by the method of Yongting et al. [25].
Data Processing
The test data were analyzed using Microsoft Excel and SPSS 15.0 software, and the significance test was performed using Duncan’s new complex range method.
Results and Discussion
Development of Morphology and Ultrastructure of Sugarcane during Smut Infection
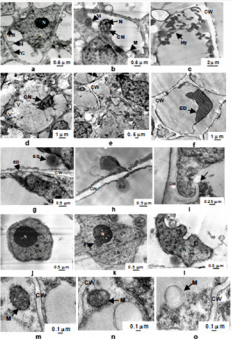
When sugar cane is infected to a certain extent by smut, the appearance of the plant will change. The morphological changes of sugarcane stem and leaves are shown in Figure 1a-b. The healthy sugarcane stems are relatively thick; the susceptible sugarcane stems are relatively small, and the internodes are long. After the main stem grows black spikes, the lateral buds also grow with the smut. Figure 1b shows the changes in sugarcane leaves. Compared with the cells of healthy sugarcane buds, obvious pathological changes occurred in the mitochondria and other cell organelles in the stalk bud cells infected by smut (Figure 2). The cells of healthy sugarcane buds are relatively regular, and there are organelles such as mitochondria, golgi apparatus and endoplasmic reticulum. The nucleus has clear nucleoli and bilayer membrane structure (Figure 2a-2j); the mitochondrial outer membrane structure is complete and elliptical shape, the ridge formed by intimal invagination is clearly distinguishable (Figure 2m). The cells of the diseased sugarcane buds were obviously deformed, and no intact organelles were observed.
The mycelium was inside (Figure 2c), the nucleus was dissolved and dispersed (Figure 2e), and the cell wall was destroyed (Figure 2i), the cells are vacuolated, the nucleus becomes small or distorted, the nucleolus becomes smaller or disappears (Figure 2b, 2d & 2f), and even the whole cell contains only some black electron dense material (Figure 2f); mitochondrial distortion (Figure 2b), or inner ridge blurring (Figure 2n), or even vacuolization (Figure 2o); significant accumulation of electron dense matter on or near the host cell wall (Figure 2g & 2h), a large number of vesicles appear in the cells (Figure 2d); compared with the nucleus of healthy sugarcane buds (Figure 2a). There is a mastoid at the edge of the nucleolus on the nucleus of Figure 2k. In Figure 2, the nucleus becomes irregular, the nucleolus has disappeared, and the chromatin distribution is uneven and degraded. There are similar findings on the external morphological changes of sugarcane caused by smut infection [26- 28], but most of them are only described by words, but no intuitive figures are used to describe them. This experiment compared the appearance and morphological changes of stems, leaves and whole plants of sugarcane healthy plants and susceptible plants. The interaction between rice and rice blast fungus, the rice plasma membrane, nuclear membrane, nucleoplasm, chloroplast and mitochondria were damaged to varying degrees after infestation by rice blast fungus, and finally the mitochondrial ridge completely disintegrated. Cavitation, chloroplast matrix sheet disappear [29].
Figure 2: Changes of smut pathogen infection on ultrastructure of bud cells in sugarcane plant. a: healthy bud cell; b-f: smut infected bud cell; g-i: cell wall in smut infected bud cell; j: nucleus in healthy bud cell; k-l: nucleus in smut infected bud cell; m: mitochondria in healthy bud cell; n-o: Mitochondria in smut infected bud cell.
Note: CW- cell wall, CN- cell nuclear, N- nucleus, ER- endoplasmic reticulum, G- golgi, M- mitochondria, ED- electron dense, V- vesicle, Pa- papillary & Hy- hypha.
Changes in Biochemical Indices of Sugarcane Infected by Smut
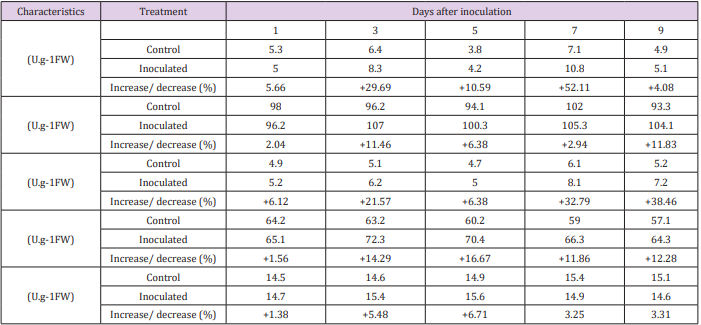
The activities of POD, SOD, PPO, chininase and β-1,3-glucanase were assessed after inoculation with smut at specific time interval. After inoculation, the activities found higher such as 4.08, 11.83, 38.46 and 12.28% as compare to non-inoculated sugarcane plant except β-1,3-glucanase activity. The highest percentage (52.11%) increased of the POD at 7th day after inoculation, SOD (11.83%) and PPO (38.46%) at 9th day after inoculation as compare to control plants. After 3rd day of inoculation, chitinase activity found maximum (14.29%) as compare to 1, 5, 7 & 9th day after inoculation. It can be seen that 1, 3 and 5th day after smut infection, β-1,3-glucanase increased, i.e. 1.38, 5.48 and 6.71% after 5th day and reduced (3.25- 3.31%) 5 to 9th day after inoculation as compare to control (Table 1). When a pathogen infects a plant, the plant cell produces a series of physiological and biochemical changes to protect against infection by the pathogen. These changes include allergic reactions after plant disease, thickening of enamel, changes in protective enzyme activity, induction and accumulation of disease-related proteins, and hormonal metabolic disorders [30].
Table 1: The effect of peroxidase (POD), superoxide dismutase (SOD), polyphenol oxidase (PPO), chitinase and β-1,3-glucanase activity of sugarcane leaves after inoculation with Ustilago scitaminea Syd.
Note: + = percent increase
Under normal condition, there are oxidative enzyme scavenging systems (such as SOD, POD etc.) in the plant, which make the active oxygen metabolism in a low level of dynamic balance, but the pathogen infects the host and causes the infected tissue. Among these defensive enzymes, SOD mainly plays a role in scavenging superoxide anion (O2-), while POD mainly removes hydrogen peroxide (H2O2) and hydroxyl radical (• OH) produced by SOD disproportionation, thereby avoiding it causes damage to cells [31]. The activity of POD in plants is significantly increased after smut infection, and the enzyme activity of resistant varieties is higher than that of susceptible varieties [32]. The results of this study indicated that the activity of POD and SOD in sugarcane increased after smut infection. Polyphenol oxidase is mainly involved in the oxidation of phenols to form strontium and the polymerization of lignin precursors. Several experiments suggested that PPO activity is positively correlated with plant disease resistance [26- 28]. Chitinase is a hydrolase that inhibits the growth of pathogenic bacteria by destroying the newly synthesized chitin at the tip of the fungal hyphae. This study found that the chitinase activity of different resistant sugarcane varieties increased after inoculation with smut and reached the highest at 3rd day after inoculation. The resistance of β-1,3-glucanase to pathogenic fungi inhibits pathogens by destroying the fungal cell wall; on the other hand, oligo-β1,3-glucanase. It is released from the fungal cell wall, thereby stimulating the expression of plant defense genes to produce a disease-resistant response [33]. This experiment showed that the activity of β-1,3-glucanase in leaves of sugarcane seedlings was higher than that of the control in the early stage of infection.
Acknowledgement
This work was supported by the fund for Guangxi Innovation Teams of Modern Agriculture Technology (nycytxgxcxtd-03-01), Guangxi Science and Technology base and Talents projects (Gui Ke AD17195100), Guangxi Key Laboratory of Sugarcane Genetic Improvement Fund (16-A-04-02).
For more Articles: https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.