External Beam Radiotherapy only as an Alternative for Radical Cystectomy in Highly Selected Bladder Cancer Patients
Introduction
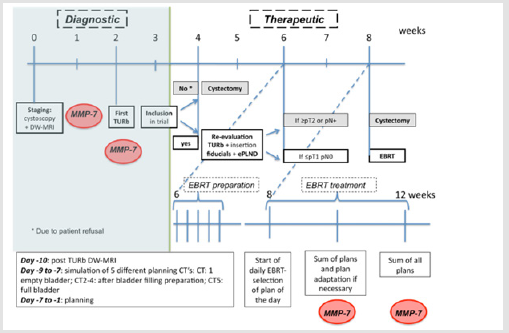
The gold standard for patients with Muscle Invasive Bladder Cancer (MIBC) is a Radical Cystectomy (RC) and extended Pelvic Lymph Node Dissection (ePLND). Peri-operative mortality rate is ± 2% and major toxicity is reported in up to 30% of the patients [1]. To increase quality of life, bladder-sparing trimodality therapies (BST) (i.e Transurethral Resection of the Bladder (TURb) + EBRT + chemotherapy) are considered a valuable alternative for RC for MIBC patients with similar local control and cause specific survival rates as reported in modern surgical series [2,3]. But also, with BST grade ≥3 toxicity has been reported in up to 36% of the patients [4]. Omitting chemotherapy reduces the risk of developing severe gastro-intestinal, urinary and haematological toxicity at the cost of worse disease-free survival [4]. In properly selected patients, re-evaluating the place of radiotherapy alone might be of interest. Therefore we initiated a phase 1 trial in which EBRT with a hypofractionated Simultaneous Integrated Boost (SIB) to the original tumor localisation was administered after complete resection of the lesion in node negative MIBC patients (Figure 1).
Figure 1: Flow chart of the treatment protocol.
Note: Within the protocol matrix metalloproteinase’s-7 (MMPs) was determined in the blood and urine as a biomarker.
Material and Methods
We designed a phase 1 trial to evaluate the place of EBRT only in highly selected MIBC patients. The ethic committee of Ghent University Hospital approved the study on 10/03/2015. The study was registered on Clinicaltrials.gov (NCT02748200). Eligible patients were patients with histological proven diagnosis of MIBC, initial stage <T3b tumours, no distant metastasis, WHO performance state 0-2, age ≥ 18 and <80 years, signed informed consent and no contra-indication for diffusion-weighted magnetic resonance imaging (DW-MRI). Before being referred for EBRT, patients underwent a second Transurethral Resection of the Bladder (TURb) in order to obtain a complete resection (defined as ≤pT1 at re-TURb) as well as an ePLND to exclude pathologically involved lymph nodes. At that time gold markers 0,4 x 5 mm (QLRAD, Netherlands) were inserted at the edges of the TURb region.
For each patient 5 different planningsCT’s were performed with 5 different bladder volumes following a standardized protocol. The clinical target volume consisted of the whole bladder and was defined on each planningsCT separately. The whole bladder was treated to 40 Gy in 20 fractions, 5 fractions/week, 4 weeks in total. Based on the summation of abnormalities seen on pre- (initial tumor region) and post TURb (zone of fibrosis) DW-MRI images, the tumor region was delineated and defined as a Gross Tumor Volume (GTV). The GTV was treated with a simultaneous integrated boost (SIB), without extending the 4-weeks treatment period. Three different dose levels to the GTV were implemented in order to increase the biological equivalent dose (BED), as MIBC has been shown to be dose-sensitive [6]. A 3+3 design was adopted. Once 1 dose level was proven to be safe (i.e. no acute RTOG >Grade3 urinary toxicity) patients were treated up to a higher dose level.
Dose prescription on 50% of the GTV was:
a) Level 1: 57.6 Gy (20 x 2.88 Gy, 5 fractions/week, 4 weeks); BED: 61 Gy.
b) Level 2: 60 Gy (20 x 3.00 Gy, 5 fractions/week, 4 weeks); BED: 64 Gy
c) Level 3: 62.4 Gy (20 x 3.12Gy, 5 fractions/week, 4 weeks); BED: 67 Gy. For all levels: BED was calculated with α/β=13 (5).
The volume of the rectum and sigmoid receiving 34.3 Gy and 34.4 Gy were constrained to 64% and 35%, to avoid grade ≥2 rectal toxicity with a probability of 90% and 95% respectively [7]. The dose received by 2% of the volume, considered as a surrogate for the maximum dose, was limited to 40 Gy for the femoral heads, small intestine and symphysis. Two sets of 5 treatment plans were generated. For one set, 5 treatment plans were optimized individually. An accumulated dose distribution was created on the third planning CT data set, with a comfortably filled bladder, using the deformation fields of deformable image registration. The other set of treatment plans were optimized simultaneously using Multiple Plan Optimization (MPO). MPO enables the specification of dose objectives on individual treatment plans and on the accumulated dose distributions. Treatment was delivered on an Elekta 18-MV linear accelerator (LINAC, Crawley, UK) with Volumetric Arc Therapy (VMAT) technique.
A daily cone beam CT was performed in order to evaluate bladder filling. After matching with the different planning CT’s the most appropriate planning was selected. After 10 sessions an accumulated dose was calculated based on the delivered plans. Considering the dose that was already delivered in daily practice during the first 10 sessions, new plans were created. In order to do so, the planning procedure was repeated for the last 10 fractions. Patients were followed weekly during therapy, 1 month after therapy and 3- monthly thereafter. At each follow up after EBRT a standard blood control was performed. In asymptomatic patients imaging (cystopscopy of the pelvis and CT thorax/abdomen) was performed 3-monthly during the first year and 6-monthly thereafter up to a period of 5 years or until progression. A DW-MRI was performed at least once after the end of radiotherapy.
The primary outcome is acute RTOG ≥Grade3 toxicity within 12 weeks after EBRT. Secondary outcome parameters are: local control (evaluated on cystoscopy and DW-MRI: if suspicious: histopathology), disease free survival (defined as period from start of EBRT till local or distant recurrence), cystectomy-free survival (defined as period from start of EBRT till salvage cystectomy), overall survival (defined as period from start of EBRT till death) and assessment of quality of life (EORTC QLQ-C30).
Results
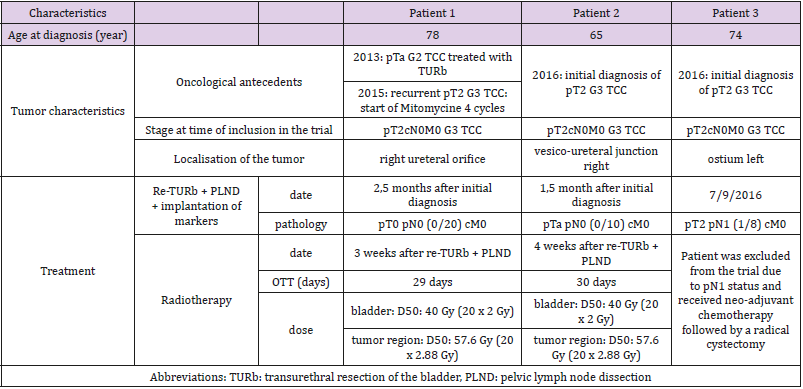
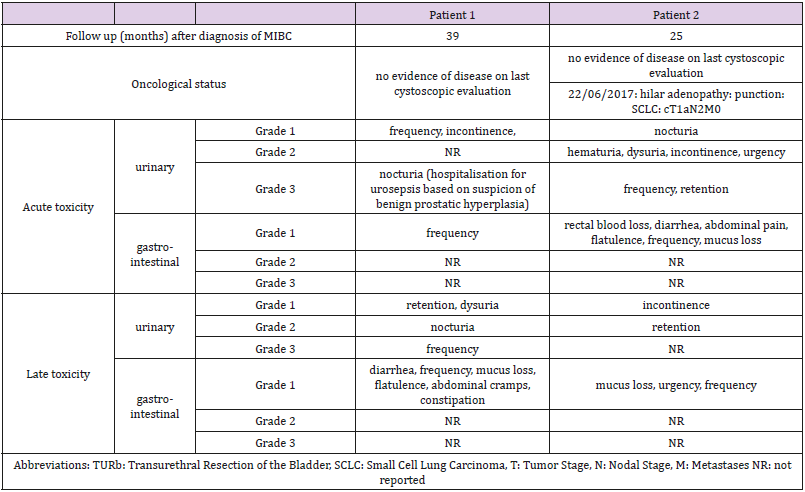
The study was closed prematurely due to poor recruitment. Three patients were included in the study. One patient was excluded due to presence of positive lymph nodes at Pelvic Lymph Node Dissection. The patients, tumor and treatment characteristics are described in Table 1. Patient’s outcome and toxicity per case are presented in Table 2. At latest follow up gastro-intestinal toxicity was limited to grade 1 constipation for the first patient and no gastro-intestinal toxicity for the second patient. Urinary toxicity at latest follow up was grade 2 nocturia and grade 1 retention for the first patient and grade 2 retention and grade 1 incontinence for the second patient.
Discussion
Proper patient selection is of utmost importance to improve the outcome of MIBC treated with BST. According to international guidelines, ideal candidates for trimodality treatment (including a TURb followed by radiochemotherapy) are patients with pT2cN0 MIBC who received a visible complete Transurethral Resection of the Bladder (TURb) and have no signs of carcinoma in situ or hydronephrosis [8-10]. In even more selected patients, chemotherapy might be omitted in order to reduce the risk of developing toxicity. Therefore, we initiated a phase 1 trial to explore the place of EBRT only with a SIB to the GTV in highly selected MIBC patients. For the latter several conditions needed to be fulfilled before patients were eligible for inclusion. First, we aimed to have a visibly complete and pathologically complete, defined as ≤pT1 disease, TURb by performing a re-TURb at time of insertion of the gold markers and PLND. The impact of a visibly complete resection on survival has been well recognized [11].
Additionally, we requested an ePLND as the sensitivity of current imaging in detecting involved lymph nodes is limited. By performing an ePLND, patients with occult lymph node metastasis were excluded from our trial. Moreover, retrospective surgical series have indicated that absence of pelvic lymph node involvement on ePLND (pN0), is a prognostic factor for cancer specific survival [12]. Additionally, an ePLND eradicates microscopic disease, which results in improved outcome both in pN+ and pN0 disease [13]. A dose response relationship has been suggested for bladder cancer. For every additional 1 Gy a gain in 5 years OS has been suggested of 7.5% [14]. In analogy to other tumour sites one can presume that tumour recurrence is most often observed in the initial tumour region [15]. Increasing the dose to that region might result in better clinical outcome without compromising toxicity [16]. In order to define this region in case of MIBC modern imaging techniques can be used. Despite promising results regarding the role of DWMRI in local and locoregional staging of MBIC, DW-MRI is not routinely performed nowadays. For local staging DW-MRI has a sensitivity and specificity of 92.3%-96% and 97.6-98.1% [17,18]. After transurethral resection, DW-MRI can differentiate between tumor recurrence and postoperative inflammation or fibrosis. The standard radiotherapy regimen for MIBC is irradiation of the whole bladder with a 2- to 3-cm margin to a dose of 60–66 Gy at 1.8 to 2 Gy fractions. However, the optimal schedule still has to be determined. Nowadays results after hypofractionated schedules are neither better nor worse than regimens of 60 Gy in 30 fractions [19].
Randomized trials comparing radiotherapy with a radiosensitizer versus radiotherapy alone have indicated a significant ad advantage for the combined regimen in terms of disease-free survival [4,20]. However, patient’s selection in our trial was far stricter. For example, only patients with a unique lesion were eligible. Also, the largest advantage of combined radiotherapy and a radio sensitizer has been observed in patients with more advanced stages that were excluded from our trial. Some trials have studied the place of TURb alone in MIBC. Although some long-term control has been described, the risk of having a relapse is substantial [21]. Therefore, we decided to perform a SIB to the initial tumor region as well as not to omit prophylactic irradiation of the whole bladder up to 40 Gy, which is probably enough to eradicate microscopic disease. It has also been demonstrated that a cystoscopic evaluation underestimates the extend of the disease discouraging an approach of not treating the full bladder [22].
The presented approach can also be an alternative for brachytherapy, which applies similar inclusion criteria [23]. With brachytherapy 5-year OS rates have been published that are comparable to the ones reported in surgical series [24]. Despite good clinical outcome and acceptable toxicity brachytherapy is not widely adopted due to lack of brachytherapy experience and logistic implications. The rapid dose fall-off as well as the accurate coverage of the tumor region with brachytherapy are major advantages in favour of brachytherapy compared to EBRT. With a plan-of the dayapproach the inaccuracy of patient positioning in case of bladder irradiation can be prevented. Studies describing adaptive planning, including a plan of the day approach, reported increased coverage of the bladder and tumor with reduction of the doses delivered to the surrounding organs at risk [25].
Also, the implementation of modern radiation techniques such as intensity-modulated arc therapy allows escalating the dose to the initial tumor region while sparing intestinal loops and rectal mucosa [26-27]. Unfortunately, our study was closed prematurely due to poor accrual. Conclusions on maximum tolerated dose can therefore not be drawn from our study. Nevertheless, the few patients that were included in our trial did not develop major toxicity and oncological outcome is excellent with more than 2 years of follow up. Therefore, our study might be considered as hypothesis generating for developing studies with high dose EBRT in well-selected MIBC patients.
For more Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.