Investigation of Folate and Folic Acid Metabolite by Validated Microbiological Methods and LC-MS/MS: Quantification in Chicken Eggs
The Importance of Folic Acid for Human Health
Folic acid (Figure 1A), pteroylglutamic acid or vitamin B9, is a water-soluble vitamin of B complex family [1]. Its International Union of Pure and Applied Chemistry (IUPAC) name is (2S)-2-[[4- [(2-amino-4-oxo-1H-pteridin-6-yl) methylamino] benzoyl] amino] pentanedioic acid. Folate is the nature form of folic acid. Due to folic acid can not be stored in the human body. Therefore, its deficiency is one of the most common vitamin deficiencies [2]. Regular intake of folic acid is essential for healthy living. Naturally, animal liver, dried beans, green leafy vegetables are all good sources of folic acid. Additionally, folic acid-rich foods in vegetables included lettuce, spinach, tomato, carrot, green cabbage, asparagus, broccoli, rape, cabbage, lentils, pods, mushrooms etc.; in fresh fruits as orange, strawberry, cherry, banana, peach, plum, apricot, bayberry, jujube, hawthorn, pomegranate, grape, kiwi, strawberry, pear, walnut etc.; in the animal food like pig liver, kidney, meat (chicken, beef, and lamb), and eggs etc.; in beans, nuts, cereals food included as soy beans, soy products, walnuts, cashews, chestnuts, almonds, pine nuts, barley, rice bran, wheat germ, and brown rice etc. [3].
Because of their low rate of consumption, folic acid deficiency in humans may lead to many diseases as megaloblastic anemia, neural tube defects in developing fetuses, cancer and heart diseases, and neurodegenerative diseases as Alzheimer’s disease and Parkinson’s disease. To avoid these risk factors, the use of folic acid fortified dietary supplements or fortified food has been increasing rapidly [1]. Folic acid-rich foods as vegetables, fresh fruits, animal food like eggs, and beans, nuts, and cereals already have been found. Folic acid supplements daily are very important to prevent some diseases induced by folic acid deficiency [4]. Folic acid-rich chicken eggs were first developed in Japan by Pharma Foods International Co., Ltd. (PFI, Japan). After the launch of the product, it was widely acclaimed in Japan. Chicken feeding technology for producing high folic acid eggs was successfully transfer from PFI to Taiwan (The Wonderful Food Co.). In Japan and Taiwan, folic acid-rich chicken eggs have already promoted. The folic acid content of chicken eggs reached 100μg/100 g egg albumin and egg yolk, which is twice the normal egg content. Because folic acid deficiency in humans may lead to many diseases. To avoid these disease occurrences, the use of folic acid fortified dietary supplements or fortified food will be continuously and rapidly increasing.
Analytical Methods for the Determination of Folic Acid
At present, a number of analytical methods have been demonstrated for the determination of folic acid in natural sources, folic acid fortified foods, and in pharmaceutical sample. These methods included thermogravimetry, spectrophotometry, high performance liquid chromatography (HPLC), HPLC coupled with mass spectrometer (MS), colorimetric, flow injection chemiluminescence, fluorometric, and electrophoresis [1]. Among of them, microbiological methods and HPLC coupled with MS were often accepted and applied to determine folic acid. In our laboratories, microbiological methods and LC-MS/MS were already successfully established to detect folate and folic acid metabolite quantification in chicken eggs.
Detection of Folic Acid Quantification in Chicken Eggs by Using Validated Microbiological Methods
Chicken eggs were purchased from the traditional markets and super markets. The whole egg (egg albumin and egg yolk) homogenized contents are used in this method followed AOAC 992.05-1995 [Total Folate (Pteroylglutamic Acid) in Infant Formula-Microbiological Methods]. The whole egg contents which are homogenized and then stored at -20 °C until further processing or can be used immediately after homogenizing. Sample preparation of homogenized whole egg was performed followed as the contents (albumin and egg yolk) of 10 eggs were collected in a clean stainless-steel homogenizer pot. These contents by using homogenizer gently were homogenized to ensure that yolk and albumin have formed visibly a homogenized mixture and avoided any foam formation. Later, the homogenized egg (egg homogenate) were distributed in self-sealing plastic pouches (20-30 mL/pouch).
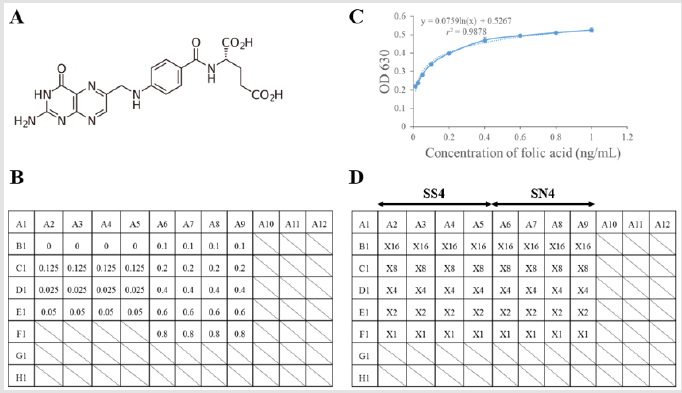
Stored the egg homogenate at -20 °C until further processing. Reagents and bacteria were required for the detection of folic acid quantification in chicken eggs by using validated microbiological methods like 0.1M phosphate buffer, sterile saline solution, 804 culture medium, Lactobacilli Inoculum (Cat. No. 05801, Nissui Seiyaku) broth, folic acid casei medium, Lactobacillus rhamnoses (ATCCTM 7469), standard folic acid solution (molecular formula and weight: C19H19N7O6 and 441.40; Cat. No. 060-01802, Wako), rat serum conjugate, and tock sample preparation. Finally, detection of folic acid quantification in chicken eggs via mixing the plate cultures uniformly by shaking plates with plate holder on vortex mixer. Care to be taken that medium inside wells should neither come to cover of plate nor in between the wells. Plates with uniformly mixed cultures be read by plate reader set at optical density (OD) at the wavelength 630nm (Figure 1). Data present that the standard curve (y = 0.0759ln(x) + 0.5267) and r2 value (0.9878) of the standard folic acid solution (Figure 1B).
Figure 1: The standard curve and microbiological method for the standard folic acid solution.
(A) The chemical structure of folic acid.
(B) The microbiological method for standard folic acid solution in the 96-well plate. The concentration (ng/mL) of folic acid was added into the each well.
(C) The standard curve and r2 value of the standard folic acid solution. Linear equation: y = 0.0759ln(x) + 0.5267; r2= 0.9878.
(D) The detection of folic acid in the chicken eggs by using the microbiological method in the 96-well plate. X: sample; Two-fold dilution for each sample (0×-16× dilution).
Quantification of Folate and Folic Acid Metabolite in Chicken Eggs by Using Validated LC-MS/MS
Chicken eggs were purchased from the traditional markets and super markets. Sample preparation of homogenized whole egg was performed followed as the contents (egg albumin and egg yolk) of eggs. The homogenized whole egg mixture was collected. Two grams of the homogenized whole egg mixture was used to mix with purification solution and proteases, then N2 gas was filled in. The mixture was hydrolyzed in a 37 °C water bath for 3 hours, then placed in a 100 °C water bath for 10 minutes, and rapidly placed in ice for 30 minutes to be returned to room temperature. Finally, to centrifuge at 15,000 rpm and 4 °C for 10 minutes and collect the supernatant through filtered procedures. Reagents were required for the detection of folic acid and folic acid metabolite quantification in chicken eggs by using LC-MS/MS like 5-methyltetrahydrofolate (5- MTHF) standard (purity ≧95%; Toronto Research Chemicals), acetonitrile (LC grade; Merck), formic acid (Merck), ammonium acetate (reagent grade, Merck), L-ascorbic acid (reagent grade, Tokyo Chemical Industry Co., Ltd.), DL-Dithiothreitol (Sigma- Aldrich), protease (reagent grade, Sigma-Aldrich), de-ionized water (18.2 ΩM) from Synergy® Water Purification System (Merck Millipore). Finally, detection of folic acid and folic acid metabolite quantification in chicken eggs via LC-MS/MS system with Agilent 1200 Series Rapid Resolution. LC (RRLC) system and Agilent 6410 QQQ liquid chromatograph tandem mass spectrometer. Data present that calibration curve (y = 141.693747x + 45.798674) and r2 value (0.99963183) for the LC-MS/MS analysis of folic acid (Figure 2); the calibration curve (y = 54.649125x – 32.362453) and r2 value (0.99893289) for the LC- MS/MS analysis of 5-MTHF (Figure 3).
Figure 2: Detection of folic acid quantification in chicken eggs by using LC-MS/MS.
(A) The calibration curve for the LC-MS/MS analysis of folic acid (5-200 ng/mL of folic acid standard). Linear equation: y = 141.693747x + 45.798674; r2 =0.99963183.
(B) 5 ng/mL folic acid of extracted ion chromatograms (EIC, m/z 295).
(C) The mass spectrum (6.593 min) of folic acid.
Figure 3: Detection of 5-methyltetrahydrofolate quantification in chicken eggs by using LC-MS/MS.
(A) The calibration curve for the LC-MS/MS analysis of 5-methyltetrahydrofolate (5-200 ng/mL of 5-methyltetrahydrofolate standard). Linear equation: y = 54.649125x – 32.362453; r2 = 0.99893289.
(B) 5 ng/mL 5-methyltetrahydrofolate of extracted ion chromatograms (EIC, m/z 313).
(C) The mass spectrum (5.319 min) of 5-methyltetrahydrofolate.
Conclusion
Successfully, folate and folic acid metabolite quantification in chicken eggs by validated microbiological methods and LC-MS/MS was established. In this study, chicken eggs were purchased from the traditional markets and super markets. The average content is 37.28 ± 17.69 μg/100 g of folic acid and 49.45 ± 10.25 μg/100 g of 5-MTHF by using validated microbiological methods and LC-MS/ MS, respectively.
For more Articles: https://biomedres01.blogspot.com/





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.