Light Emitting Diodes as Alternative Light Sources: Effects of Ultraviolet Frequencies on Microbial Replication
Introduction
Hospital acquired infections (HIAs) are a major global problem as they increase morbidity, mortality, hospitalization length and the cost of care for hospitalized patients [1,2]. Several pathogenic microorganisms including Pseudomonas aeruginosa, E. coli, Enterococcus spy (including VRE) and methicillin-resistant Staphylococcus aureus are able to remain on medical surfaces and devices for long periods [3,4]. Studies carried out by Weber et al. [5] show that about 20-40% of HIAs are due to the lack of hygienic attention of health care workers who may have become contaminated through direct infection with the patient in hospital or indirectly by touching the contaminated environmental surfaces. In fact, the environment is increasingly recognized as a significant element for microbial cross-contamination [6]. For this reason, a report by the CDC highlights the need to not underestimate the standards of good hygiene practice, which, although obvious, remain a reference element [7]. Among the methods of disinfection, the use of disinfectants is another element to be considered in order to implement a proper sanitization of environments and objects. In addition, the use of ultraviolet radiation is also becoming more and more emerging [8].
The radiation with the most germicidal effect is represented by UV-C (200-280 nm). It has long been known that the damage that these determine is given by the formation of bonds between the pyrimidine nitrogenous bases present in the DNA of microorganisms that combine to form dimers of thymine that if they remain prevent microorganisms from being able to replicate [9]. Such radiations are artificially produced by the classic germicidal lamps. Recent researches have shown that other electronic frequencies can have a biocidal effect. Also, the appearance of alternative light sources: Light Emitting Diodes (LEDs) allows you to select the frequencies of interest to capture the effects of these on various species of microbes [8,10]. In view of this, it is necessary to examine the issue in greater depth in order to be able to consider its application in the health environment. The purpose of this work is to verify the biocidal effect of the various UV frequencies (A-B-C) emitted by LEDs on some microbial species, relevant in HIAs, at different exposure times (10-30-60 minutes).
Methods
This research is a pilot study in which pre and post exposure phases were performed at different LED sources, with the following frequencies: 276 and 279 nm (UV-C) by CUD7GF1A; 306 and 308 nm (UV-B) by LEUVA66G; 305 and 306 nm (UV-B) by CUD1AF4C; 343 and 354 nm (UV-A) by CUD4AF1B. The survey was conducted in January and February 2018 in the Laboratory of Environmental Hygiene (microbiological section) of the University of Siena.
Experimental Setting
In order to conduct and standardize the experiments it was necessary to build a setting that would allow a uniform distribution between the light source and the Petri dishes. The setting to conduct the study was designed with the help of the 3D modeling program, Solid works, and then realized with a 3D lithographic printer, Form lab Form 2. Our setting had two supports, shaped as an inverted cone, in which the vertex had a hole specifically created to allow the insertion of the various LEDs. Those LEDs were soldered on PCBs and powered in order to generate 3 mW. To standardize this power and measure the different wavelengths emitted by the LEDs, an integrating sphere connected to a spectrophotometer was used.
Selection of Microorganisms and Preparation Protocol
The microorganisms were chosen on the basis of the following features
a. Morphological characteristics
b. Ability to produce spores (forms of resistance)
c. Causation of HIAs.
They were: Staphylococcus aureus ATCC13150; Pseudomonas aeruginosa ATCC27853; Escherichia coli (not ATCC); Bacillus subtilis (not ATCC); Enterococcus faecalis ATCC51299. In the case of Bacillus subtilis the inoculation was on Nutrient Agar with the addition of a Manganese chloride solution. The incubation of the Petri dishes took place at a temperature of 30°C and lasted for about 10 days until the complete transformation of the microorganisms in sporigenous form. For the spores of Bacillus subtilis and for all other microorganisms in the study, bacterial suspensions were set up in 10 ml of sterile Phosphate Buffered Saline (PBS) in order to obtain a concentration of 0.3 McFarland. For each bacterial and sporigenous suspension dilutions were made and for the present work a dilution of 10-2 was used.
Rates of 40μL of these dilutions were sown in duplicate by spatula in 55 mm diameter Petri dishes in the agar specific medium. For Staphylococcus aureus (Mannitol Salt Agar); Pseudomonas aeruginosa (Cetrimide agar); Escherichia coli (Brilliance E. coli/ Coliform Selective Medium); Bacillus subtilis (Nutrient Agar); Enterococcus faecalis (Slanet And Bartley Medium). The Petri dishes thus contaminated were exposed to the radiation of the various LEDs. Each Petri dish was individually mounted in the cone inversed setting, and exposed to a specific UV wavelength at 10, 30 and 60 minutes. Subsequently, the irradiated plates were incubated in a thermostat at 36°C, for each of them reading was made at 24 hours. For each microorganism, four Petri dishes contaminated but not exposed to UV radiation were used as controls. They were also incubated in a thermostat at 36°C and then read at 24 hours.
Results
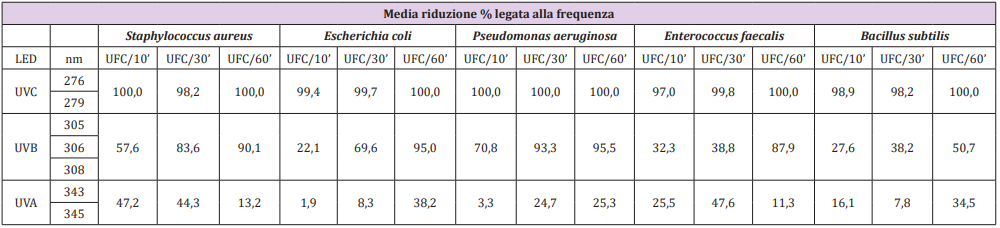
Differences in the effectiveness of LEDs in reducing the microbial load have been observed, from UV-C frequencies to UV-B and eventually to UV-A. The average reduction percentage linked to the frequency of UV-C LEDs was 100% already at 10’ for Sthaphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa, while for Enterococcus faecalis there was a reduction of 97% and 99% for Bacillus subtilis. After 30’ of exposure for Sthaphylococcus aureus and Bacillus subtilis there was a reduction of 98.2%, for Escherichia coli of 99.7%, for Enterococcus faecalis and Pseudomonas aeruginosa of 100%. All the microorganisms tested after 60’ of exposure showed, instead, a reduction of 100%. The average percentage of reduction related to the frequency of UV-B LEDs was lower than the results obtained by testing UV-C rays, the microbial charge decreased as the exposure time increased. In particular, it emerges that Pseudomonas aeruginosa was most affected by their effectiveness, and at 10’ it was already reduced by 70.8% and 95.5% at 60’, while the microorganism that was less sensitive was Escherichia coli, with a reduction of 22.1% at 10’ and 95% at 60’. The average percentage of reduction related to the frequency of UV-A LEDs is the lowest compared to the others mentioned above, but still gave good results compared to the controls, although with certain critical issues, especially for Sthaphylococcus aureus, Enterococcus faecalis and Bacillus subtilis. Table 1 shows the results of the specific species-frequency pairings.
Discussion
The results of testing LEDs at different wavelengths show that their effectiveness depends on the dissimilar sensitivity of microorganisms to ultraviolet light and on the time of irradiation, which results in a progressive reduction of the microbial load. This is consistent with most microorganisms [11]. Recent study shows that an effect that alters DNA replication is not only dependent on UV-C (200-280 nm), but also on UV-B (280-320 nm). [10] Our results have shown that UV-C, UV-B and UV-A have a reducing effect on the microbial load, albeit with important differences between them. The penetration capacity of UV-A and UV-C is different; the former does not induce direct damage to DNA, capable of forming thymine dimers, [12] but damage cellular proteins, induce the formation of reactive oxygen species such as singlet oxygen and hydrogen peroxide as also claimed by Hargreaves A et al. [13]. Noteworthy is the fact that while the DNA damage induced by UV-C can be repaired by a photolytic enzyme [14], those reported by UV-A cannot be healed in any way, because the enzymes used for the repair are damaged. Our results have shown that UV-A, although to a lesser extent, partially inhibits bacterial replication. In this case, such a reduction could be assumed to be due to this mechanism.
The possibility of associating the effects of both UV-A and UV-C with bacterial replication is currently a field in which several studies are being conducted. Akgün M.P. and collaborators have recently conducted a study where the combination of UV-C and UV-A has allowed a greater effectiveness in reducing the microbial load [10,15]. In our study for Staphylococcus aureus, Enterococcus faecalis and Bacillus subtilis, critical issues were found. The number of colony-forming units (CFU) was higher than the longest exposure times to UV-C and UV-A, probably due to a sampling problem, possible limit of this pilot study. Moreover, almost all the Petri dishes showed a contamination on the circumference borders of them, probably due to their direct contact with the cone rested in the on the medium. This was particularly evident with the experiments conducted with UV-C, in which these contaminations were very sharp due the inability of the radiation to reach the resting surface of cone circumference. However, this limit could be useful to confirm a positive sowing of the Petri Dishes. This observation leads to a probable underestimation of the results, which could have had a greater percentage reduction. This small systematic inconvenience was controllable and allowed us to think about possible improvements in the experimental setting. In fact, assuming to use of 90 mm Petri dishes, instead of 55 mm in diameter, we could have, for each plate treated, a matched control of itself, in the external part of the Petri dishes.
Conclusion
The results obtained in testing the efficacy of LEDs at different wavelengths are consistent with what has been reported in recent literature. In particular, it is stressed that the greatest action with a germicidal effect is performed by UV-C rays (200-280 nm) [10], but similarly other frequencies of the light can be useful to reduce the microbial replication although efficacy decreases at increasing wavelength, progressively, from UV-C, UV-B (280- 320 nm) and finally UV-A (320- 400 nm). Moreover, the hypothesis of being able to combine and control wavelengths emitted by UV-A, UV-B and UVC suggests the possibility of having a greater effectiveness in inhibiting microbial replication by exploiting its different properties. Also, the use of these innovative sources, although still very “young” and have a large margin for improvement, can be found in numerous disinfection application [6,16,17]. LEDs have many advantages over UV lamps, including small size; impact resistance; no need to heat up to operate; low energy consumption; longer life than germicidal lamps; no mercury content; but most importantly, they emit multiple individual wavelengths [10,15]. Such an eventuality opens in fact innumerable hypotheses of study and possible applicative relapses.
Acknowledgement
We want to thank EBV Elektronik (Dr. Pierluigi Rossetti) and Seoul Viosys for providing us samples of LEDs.
For more Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.