Selective Estrogen Receptor Modulator Raloxifene as a Possible Regulator of Arginine Vasopressin
Introduction
In general, the average life expectancy is increasing worldwide therefore women spend more than one- third of their lives in estrogen deficiency. Several studies confirmed that cardiovascular diseases appear more likely in women over 60 years old than age-matched men [1]. It is also proved that middle-aged women who receive hormone replacement less likely to suffer from circulatory disorders. Although large number of animal studies confirmed the protective effects of estrogen replacement, human clinical researches were inconclusive. Long term hormone administration increases the chance of breast and endometrial carcinoma development [2]. However selective estrogen receptor modulators (SERM) are a class of drugs that have estrogen agonist characteristics in target tissues such as cardiovascular system or central nervous system, they are not oncogenic [3].
The most widely used SERM is raloxifene (RAL), which is proved to have several beneficial effects on the level of lipoprotein(a), serum homocysteine and C-reactive protein [4]. Moreover, it inhibits the lipid peroxidation of blood vessels, platelet aggregation and myointimal proliferation, conclusively the incidence of atherosclerotic processes [5]. Estrogen bound to its receptor modulates the release of arginine vasopressin (AVP), the key molecule in pathogenesis of hypertension and for this reason SERMs can be a potential treatment for postmenopausal women. Hence, the aim of our study was to investigate the possible cardioprotective effect of E2/RAL treatment throughout the reduction of AVP.
Materials and Methods
Experimental Protocol
During our experiments female rats weighing 230-250g were used. Animals were kept under standard housing conditions (temperature, light). After one-week acclimatization, animals were randomized into the following groups: fertile female rats (control, CTRL), ovariectomized (OVX), OVX + estrogen (E2), OVX + raloxifene (RAL). In order to achieve estrogen-depleted conditions surgical ovariectomy was performed. During ovariectomy the ovaries on both sides were clamped and removed under anesthesia. After one month of regeneration, the animals were treated with E2 (0,10mg/ kg/day, per os) or with RAL (in three different doses: 0,11mg/kg/ day; 0,33mg/kg/day; 1,0mg/kg/day, per os) for two weeks. All procedures were approved by the Institutional Ethical Committee and were performed in accordance with the guidelines of the European Community.
Measurement of Plasma AVP
The level of arginine vasopressin (AVP) was determined with Radioimmunoassay (RIA) based on a previously described method [6]. A synthetic AVP (Organon, Oss, The Netherlands, antidiuretic activity 408 IU/mg) was used as a reference for antibody production and radio labelling. For immunization animals were received 1,0mg immunogen emulsified in 1,0ml Freund’s adjuvant. The 125I labeling of vasopressin was performed by chloramine T method, then the labeled hormone was purified with reverse phase chromatography. The specific activity of 125I labeled vasopressin was between 49,90 and 61,10 TBq/ mmol. Blood samples were collected after decapitation, stored in polystyrene tubes (1,40mg Na2EDTA, 30,0μl isotonic NaCl). After 10 seconds of centrifugation (1000g, 4°C), samples were stored at -20°C until further measurements.
RIA determination was performed 72 hours after sample collection. Vasopressin extraction was carried out from plasma samples with Amprep C8 minicolumn (RPN 1902, Amersham, Buckinghamshire, United Kingdom). Standard curves were within the range 1,0-128,0pg/assay tube. Reference vasopressin-free dilutions were extracted from homozygotic rats suffered from diabetes insipidus (CPB-TNO, Zeist, The Netherlands). The dry residue was re-dissolved in 125μl of RIA buffer, then 50μl aliquots were used for the RIA in duplicate. The sensitivity of RIA was 1pg/ tube. The level of plasma vasopressin is given in pg/ml.
Statistical Analysis
The results are expressed as means ± S.E.M. Statistical analysis was performed with Mann W. test. A probability level of ≤ 0.05 was considered as a significant difference.
Results
The Effects of Estrogen and Raloxifene on Plasma AVP Level in OVX Rats
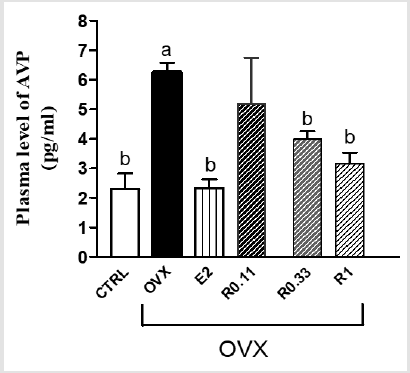
Figure 1 shows that OVX caused a significant increase in plasma AVP level in comparison with fertile females. Administration of E2 (0,10mg/kg/day, per os) or RAL (in three different doses: 0,11mg/ kg/day; 0,33mg/kg/day; 1,0mg/kg/day, per os) decreased the level of plasma AVP in OVX rat. Additionally, RAL treatment reduced the AVP level in a dose dependent way.
Figure 1: Results are shown as means ± S.E.M, n=10.
Note: Statistical significance: ap: <0.05 compared to fertile CTRL rats, bp: <0.05 compared to OVX group
CTRL= control fertile female rats, OVX= surgical ovariectomy, E2: estrogen treatment, R 0.11/R0.33/R1= 0.11mg/kg, 0.33mg/ kg, 1mg/kg raloxifene treatment, AVP= arginine vasopressin.
Discussion
Our findings show that E2 and RAL have an important role in the regulation of AVP level. Here, we demonstrated, that OVX resulted in a significant increase in the level of plasma AVP. Our findings are in accordance with a previous study that proved the increasing activity of AVP neurons in postmenopausal women [7]. We also proved, that this increased AVP content could be strongly reversed with E2 or RAL treatment. It has been reported earlier that RAL reduces blood pressure in hypertensive OVX rats [8]. These data are in line with a previous research by Daniela Grassi et al. [9], who found that AVP expression is highly regulated by raloxifene through the activation of G protein-coupled estrogen receptor-1 in neuroblastoma cells. These results suggest that RAL has a significant role in the regulation of blood pressure. As a result of hormone deprivation, postmenopausal women tend to have a higher prevalence of hypertension which is the major risk factor of cardiovascular disorders [10]. As RAL has an estrogen-agonist effect on lipids and cardiovascular markers but acts as an estrogen antagonist in breast and uterus, therefore its administration demonstrates a potential therapeutic strategy for postmenopausal women.
Acknowledgement
This work was supported by the Ministry of Human Capacities 20391-3/2018/FEKUSTRAT, GINOP-2.3.2-15-2016-00062 and the European Union, co-financed by the European Social Fund (EFOP- 3.6.2-16-2017-00009).
For more Articles on: https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.