Tumor Necrosis Factor-α, Lactate Dehydrogenase and Gamma Glutamyl Transferase as Independent Predictors for Monitoring HCV-Chronic Liver Disease
Introduction
Hepatocellular carcinoma (HCC) ranks as the 5th most common malignant cancer and the 3rd most frequent cause of cancer leading death worldwide [1-3]. HCC is an environmentally related cancer, with both viral and chemical carcinogens involved in multi stage process [4]. However, the reasons for viral persistence and transformation from acute to chronic infection are not clear, but it is known that both viral and host characteristics can influence the outcome of the infection [5]. The host response to hepatitis viruses involves various components of the immune system, including T-lymphocyte immune-regulatory cytokines [6,7]. TNF-α, a monocyte/macrophage-derived cytokine, is known to possess antineoplastic, anti-viral, and potent immunomodulatory activities [8]. It has been reported that TNF-α is involved in the pathogenesis of a diversity of liver diseases including viral hepatitis and HCC [9]. The majority of HCC patients are not amenable to curative therapy as they are detected at late stages [4].
Therefore, several tumor markers are used currently for the evaluation of tumor progression and prognosis of patients with HCC including AFP, Lens Culinaris agglutinin A-reactive fraction of AFP (AFP-L3) [10]. However, AFP is a fairly specific but insensitive marker for HCC; therefore, to improve the sensitivity of HCC detection, various markers are used in combination with AFP [11]. GGT is a microsomal enzyme present in hepatocytes and biliary epithelial cells, renal tubules, pancrease and intestine [12]. It is generally accepted as the most sensitive marker of cholestasis and pancreatic disease or enzymatic induction by alcohol and drugs [13]. However, GGT activity is not necessarily considered a routine test in the evaluation of liver disease because it is believed to contribute little diagnostic information [14-16]. Lactate dehydrogenase (LDH), which is a key enzyme in the conversion of pyruvate to lactate under anaerobic environment [17], has been recognized as an indirect marker of the extent of tumor hypoxia a key biological mechanism for the development of treatment resistance in cancer cells [18,19]. It was known that LDH had 5 isoenzymes, and each of them might function differently in the tumor progression [20]. Therefore, this study was conducted to evaluate the clinical significance of TNF-α level and its correlation with the activity of LDH and GGT in HCVprogressive liver disease.
Materials and Methods
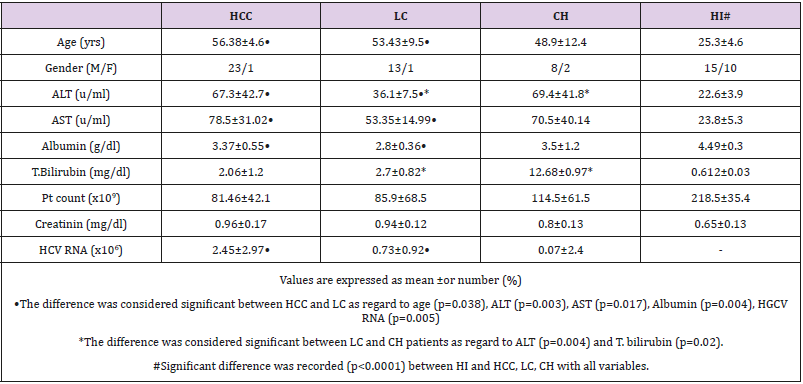
Forty-eight (44 men and 4 women) patients suffering from HCVchronic liver diseases admitted at Gastroenterology surgical center, Mansoura university, Egypt were included in our study. All patients had positive reactivity for HCV Abs with detectable HCV RNA and with no serologic evidence of co-infection with other hepatotropic viruses or human immunodeficiency virus. Twenty-five (15 men and 10 women; mean age 25.3±4.63 yrs, range 19-34 yrs) healthy individuals (HI) served as control group were selected without a clinical history of hepatitis and without symptoms or signs of liver diseases. HCV-infected patients were histopathologically diagnosed and accordingly divided into: 24(50%) patients with HCC (23 men and 1 women; mean age 56.4±6.4yrs; range 50-66 yrs); 14(29.2%) patients with liver cirrhosis (13 men and 1 women; mean age 52.6±9.14yrs, range 30-61yrs) and 10(20.8%) patients with chronic hepatitis (8 men and women; mean age 48.9±12.4 yrs; range 26-65yrs). Serum samples of all individuals were collected and stored at -70°C until used. All sera were investigated for ALT, AST, Albumin, T.bilirubin, LDH and GGT using Hitachi 750XRC Analyzer. Serum AFP was detected using AFP kit (Abbott Laboratories, USA) and the results were automatically calculated using 1Mx Abbott equipment. In vitro human TNF-alpha ELISA kit (RayBiotech, Inc., www.raybiotech.com) was used for the quantitative measurement of serum TNF-alpha.
Table 1: Demographic and base line characteristics of patients with HCV-chronic liver disease and healthy individuals.
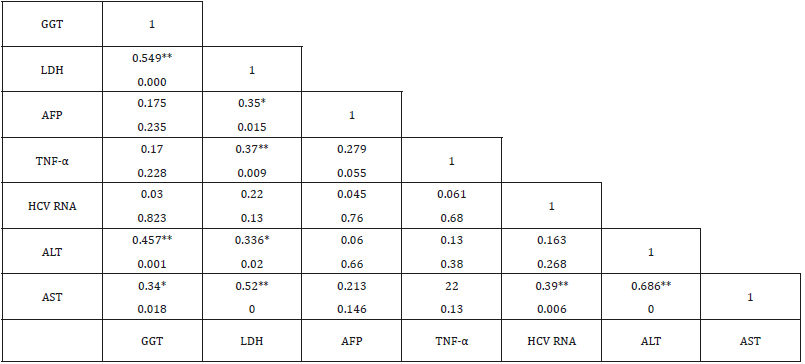
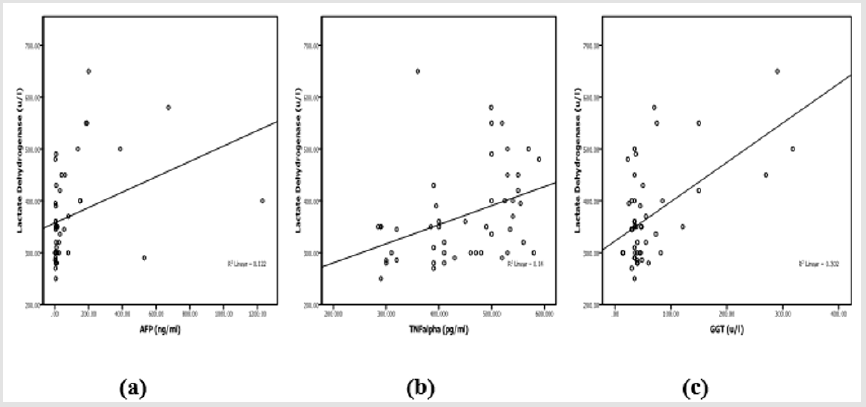
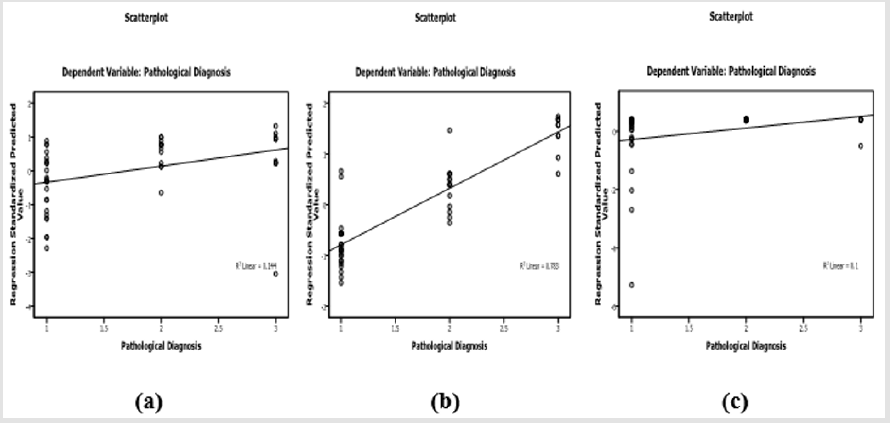
Laboratory investigation and biochemical markers in HCVinfected patients considered to viral load (data not shown) showed no significant difference with all variables within all groups except in LDH group (388.5±130.56 vs 271.25±15.47, p=0.01). Correlation between TNF-α, LDH, GGT and AFP in HCV-infected patients was listed in Table 3. AFP was associated with LDH (r=0.35, p=0.015), TNF-α was associated with LDH (r=0.37, p=0.009), GGT was associated with LDH (r=0.549, p<0.0001), ALT (r=0.457, p=0.001) and AST (r=0.34, p=0.018). Therefore, LDH showed significant correlation with AFP, TNF-α, GGT (Figure 1) in addition to ALT (r=0.336, p=0.02) and AST (r=0.52, p<0.0001). LDH (p=0.008), TNF-α (p<0.0001) and AFP (p=0.028) showed significant prediction for disease progression in HCV chronic liver disease (Figure 2) by linear regression analysis.
Table 3: Correlation between TNF-α, LDH, GGT and other biochemical markers of liver diseases in HCV-chronic liver disease.
Note:
a) **Correlation is considered significant at the 0.01 level.
b) *Correlation is considered significant at the 0.05 level.
Figure 1: Correlation between LDH with AFP
a) TNF-α
b) And GGT
c) In patients with HCV- chronic liver disease.
Figure 2: Scatterplot of LDH
a) TNF-α
b) And AFP
c) With disease progression in patients with HCV-chronic liver disease.
Discussion
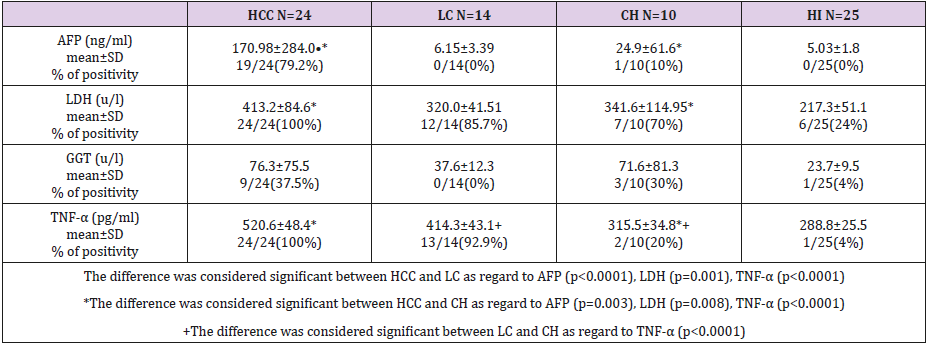
Several serum tumor markers are used currently for the evaluation of tumor progression and prognosis of patients with HCC, including AFP [10]. The diagnostic capacity of AFP depends on its elevation in the serum; concentration of AFP greater than the upper reference limit indicate the presence of HCC but values below this level are less useful because they may also occur in chronic liver disease [11]. In the present study, serum concentration of AFP in HCC group with average value 170.98±284.0 ng/ml was considered significant (p<0.0001) compared to LC (6.15±3.39ng/ml) and CH (24.9±61.6ng/ml, p=0.003). Positive serum AFP (>15ng/ml) was detected in 79.2% of HCC patients and in only one case of CH (10%) but all cases with liver cirrhosis were detected negative for AFP. Therefore, the sensitivity of AFP in HCC patients in current study is comparable to that recorded by Raedle et al. [21] and Gadelhak et al. [11] (69.3%, 58.46% respectively). However, this low sensitivity of AFP makes its value limited in the diagnosis and the prognosis of HCC, but it is independent of other predictors [22].
Therefore, to improve the sensitivity of HCC detection by serum markers, various biomarkers are used in combination with AFP.TNF-α plays a central role in the host’s immunomodulatory response to infective agents [23] and hepatitis infection is associated with increased transcriptional expression of the TNF-α gene in the liver with high serum levels of TNF-α [24]. Currently, the level of TNF-α was elevated significantly (p<0.0001) in the sera of HCC patients compared to LC and CH and also, elevated in LC than CH (p<0.0001). Elevated serum TNF-α level have been observed by other researchers even in patients with mild liver inflammation, indicating that this cytokine could be used as a predictor of liver inflammation [25]. TNF-α level was not correlated with ALT, AST, or viral load in current study and these results are in consistence with other reports, that, serum TNF-α level was not correlated with serum ALT or AST activities neither in HBV nor in HCV infected patients [26].
Such results have also been reported by other authors and it is concluded that, measurement of TNF-α levels reflects liver injury despite normal levels of liver enzymes [27,28]. Increased GGT activity is associated with liver injury and with mortality in the general population but less is known about its association with chronic hepatitis (HCV) outcomes [29]. Among patients with chronic HCV, higher GGT activity has been associated with more severe liver disease in a number of cross sectional studies [30-34]. On contrary, other cross sectional studies provide in conclusive evidence that GGT is associated with liver disease progression [35]. GGT elevation in current study was increased in HCC patients than in LC and CH but significant association with liver disease progression was not detected suggesting that GGT is not a marker for disease severity.
Patients with elevated GGT had significantly higher serum levels of AST but no association between ALT, viral load and GGT [36]. Also, Silva et al. [32], demonstrated that there is no significant association between increased GGT levels and ALT in logistic regression analysis. In current study, GGT showed significant association with ALT (r=0.457, p=0.001) and AST (r=0.34, p=0.018) but there is no significant association with viral load. These results are in agreement with Hwang et al. [37] who demonstrated that, a highly significant correlation was recorded between serum level of ALT and GGT but elevated serum GGT was not correlated with serum HCV RNA titer or HCV genotype. Such results suggest that the histological damage could represent a common origin of the alteration of both enzymes ALT and GGT [38]. LDH is a commonly used serum biomarker, which is easily and cheap to detect and, thus, appropriate for the use in routine clinical practice [19]. In current study, serum concentration of LDH was highly elevated in patients with HCC than in LC (p=0.001) and CH (p=0.008) but there is no significant difference between LC and CH. Therefore, elevated LDH is a possible indicator of disease progression [39]. LDH was also correlated positively with liver disease markers ALT (r=0336, p=0.02) and AST (r=0.52, p<0.0001) suggesting that, LDH is an enzyme that is expressed at higher levels when cells are distressed and damaged. LDH, TNF-α, and AFP showed significant prediction for disease progression in HCV infected patients of our study. Therefore, our results concluded that, LDH and TNF-α could be used simultaneously with AFP for the evaluation of chronic inflammation associated with HCV infection leading to HCC development.
For more Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.