Acute Toxicity of Copper and Zinc and their Lethal Concentration on Clarias Gariepinus (Cat Fish)
Introduction
Fish are particularly vulnerable and heavily exposed to pollution in their aquatic environments where they live and feed, because they can hardly escape from the detrimental effects of pollutants [1,2]. In comparison with invertebrates, Fish are convenient test subject for indication of ecosystem health as they show higher sensitivity to many toxicant [3-6]. Owing to this, Fish are considered to be most significant bio-monitors in aquatic ecosystem for the estimation of metal pollution level [7-8]. They offer several specific advantages in describing the natural characteristics of aquatic system and in assessing changes to habitats [9]. In addition, fish are located at the end of the aquatic food chain and may accumulate metals and possibly pass them to human beings through food causing chronic or acute disease [10].
Heavy metal pollution In aquatic environment, often results from direct atmospheric deposition, geological weathering or through the discharge of agricultural, municipal, residential or industrial waste products, and also through waste water treatment plants [11-14] for instance, Coal combustion is one of the most important anthropogenic emission sources of trace elements and an important source of a number of metals [15,16] noted that Increasing human population, rapid industrialization and commercial activities in Onitsha metropolis and the resultant continuous discharge of domestic and industrial effluents into the Niger river accounts for the increase in concentration of heavy metal in the river. which also resulted in bioaccumulation of heavy metals in the fish macro bacterium Rosenberg caught in the river [17]. Heavy metals pollution is one of the five major types of toxic pollutants commonly present in surface and ground waters [18]. Fish accumulate pollutants preferentially in their fatty tissues like liver and the effects become apparent when concentration in such tissues attain a threshold level [19].
However, this accumulation depends upon their intake, storage and elimination from the body [20]. This means that metals with high uptake and low elimination rates in tissues of fish are expected to be accumulated to higher levels [21,22]. Heavy metals can be taken up into fish either from ingestion of contaminated food via the alimentary canal or through the gills and skin [23,24]. Effectively after the absorption, metals in fish are then transported through blood stream to the organs & tissues where they are accumulated [25,26]. Many factors can affect or modify the degree of sensitivity shown by an organism to different toxicants. Some examples are the specific diet, season of the year, and water quality variables such as temperature, ph, and hardness [27]. Since fish are poikilothermic, temperature is one of the most important environmental factors controlling biological rates, and therefore would be expected to influence tissue uptake of metals as well as the toxicity tolerance in fish [28]. An increase in water temperature will increase the rate of chemical reactions in fish as predicted by the Arrhenius theory [29]. This theory also predicts that the toxicity of a metal also increases with a rise in temperature as reported during exposure of Tilapia zilli and Clarias lazera [30].
The calcium content (hardness) of water acts as a modifying factor with an increase in metal toxicity in water with a low calcium content [31,32]. The toxicity of pollutants may be increased by a reduction in dissolved oxygen [33] whilst the hydrogen ion concentration (ph.) can affect the ionization and the solubility of the metal [34]. The toxicity of metals causes negative biological effects on survival, activity, growth, metabolism, or reproduction of many species. Symptoms of metal poisoning typically include hyperactivity followed by sluggishness before death, swimming at the surface, lethargic and uncoordinated movements hemorrhage at gills and base of fins, shed scales, and extensive body and gill mucus [35]. Element which are essential under normal circumstances, such as copper, and zinc may become pollutant when excessive amount is present and exhibit toxic effect on organisms. Therefore, the establishment of critical concentration of these elements in aquatic systems, whether natural or man-made, by bio-assay techniques (toxicity tests) is necessary in determining acceptable levels of pollution [36]. Ecological needs, size and age of individuals, their life cycle, feeding habits and the season of capture were also found to affect experimental results from the tissues [37-39].
There have been numerous studies on the toxicity of heavy metals (copper and zinc) and their lethal influence in aquatic ecosystem. Abadi et al. [40] noted that Copper (cu), and Zinc (zn), are essential metals with known important biological roles. Their toxicity occurs either at metabolic deficiencies or at high concentration [41] and the form in which they occur in water influences their toxicity to fish. for instance, the ionic forms of metals or simple inorganic compounds are more toxic than complex inorganic or organic compounds. The toxicity of copper and zinc to aquatic life varies with the physical and chemical conditions of the water. Factors like water hardness, alkalinity, ph., dissolved oxygen and temperature affect the toxicity of copper and zinc [34,42,43].
There are also, marked differences in copper and zinc sensitivity among species. For instance, [44] reported that copper was significantly more toxic to Oreochromis niloticus than the Clarias gariepinus, the 96hrs LC50 values for Oreochromis niloticus and Clarias gariepinus were revealed to be 58.837mg/l and 70.135mg/l respectively. Also, high concentration of copper has been reported to inhibit catalase enzyme in liver, gill and muscle after 24hrs of exposure in Cyprinus carpio [45]. On the other hand, spear (1981) reported that the order perciforms were found to be more resistant while clupeiforms are more sensitive to zinc. According to Cusimano et al. [43] Salmonid fishes were found to be highly susceptible to zinc intoxication in soft water and the 96hrs LC50 value for the rainbow trout was found to be 0.066mg/l. However, Sobbe and De (1974) noted that the value goes up to 2.5 to 4.7mg/l in very hard water.
Non-salmonid fish on the other hand are known to be less sensitive than salmonid fish. Khangarot et al. [46] noted 96hrs LC50 for common carp in soft water to be 3.12mg/l. The main target of water borne copper and zinc toxicity are the gills, kidney, liver and skeletal muscles [46,47]. Copper induced histological alterations are found in the gill, kidney hematopoietic tissues, mechanoreceptors, chemo receptors, and other tissues Sorensen (1991). Higher doses of copper caused visible external lesions such as discoloration and necroses on livers of Cyprinus carpi, Corassius auratus and Corydoras paleatus [48]. Clarke et al. (1981) reported that zinc toxicity disrupts the ca2+ uptake in the gills, leading to hypocalcaemia and eventually death [49]. Copper and Zinc toxicity are known to impair growth, reproduction, hatching, reduced survival of young, and other effect in fish [50,51]. On the combined effect of Copper and Zinc, Obiakor et al. [44] evaluated the genotoxicity of copper, and zinc and their binary mixture on Synodontis clarias and Tilapia nilotica using micronucleus test in fish genome.
The authors documented elevated micronuclei frequency following exposure to highest and lowest concentration of binary mixture of the heavy metals on the fish species at 96hrs LC50. Hilmy et al. [30] reported that the acute toxicity to juvenile Clarias lazera of a mixture of copper and zinc over a 96hrs exposure period showed The comparison between metal residue in fish that the uptake of one metal was decrease by the presence of the other, Copper had more toxic effect than zinc. The importance of modifying factors cannot be overestimated, as many records of their influence on the variation in toxicity can be found in the literature, however it is vital to determine the specific LC50 value (Lethal Concentration for 50% of the test organism) for a given combination of abiotic conditions. The study reported here was carried out to determine the acute toxicity and 96hrs lethal threshold concentration or incipient LC50 values of copper and zinc at the selected temperature of 25±2oC for fingerling of Clarias gariepinus. The lethal threshold concentration is usually defined as that concentration which causes death to half the test organisms with a specified period of time. eg 96hrs [52].
Materials and Methods
150 Clarias gariepinus fingerlings were obtained from a local fish farm in awka, Anambra State. They were placed in five plastic aquaria filled with underground well water. The aquaria were covered with net to prevent escape of the fish. The fish were fed after 24hrs of arrival with commercial feed. They acclimated to the experimental site condition for 12 days at the selected temperature of 25±2oC. The mean initial weight of each fish was 4g ± 2 and the length were 8.5cm ± 1.5. Underground well water was used for this experiment. The water was obtained from an underground well at the Animal and Fishery unit, Nnamdi Azikiwe University, Awka. Water quality parameters such as temperature, dissolved oxygen, pH, and hardness were measured before the commencement of the study. The compounds used in the experiment were copper sulphate (CuS04) and zinc sulphate (ZnS04) salt. The salt of zinc and copper dissolved separately in water were used as toxicant. The test organisms were subjected to different concentrations (40, 60, 80, 100 mg/l) of the zinc sulphate and copper sulphate respectively. The volume of each aquarium was measured before the calculated concentrations of zinc salt and copper salt were added.
The acclimated fish were not fed 24h before the start of the tests. Also, the fish were not fed during the course of the experiment. The experiment lasted for 96h, the number of dead fishes were counted every 24h and were removed as fast as possible. The fish became dead when it was motionless and sank to the bottom of the aquarium. The mortality rate was determined at the end of 96hrs. Simultaneously the control group was kept in the experimental water. There was no addition of copper salt and zinc salt to the group. The mortality on the control did not exceed 5%. The fish were in good condition except for a slight decrease in weight. Mixed concentration of copper and zinc were also tested to know the toxicity difference between the two salts. This was done by addition of 1/10 (one tenth) the LC50 (lethal concentration) of the two salt respectively. The procure was carried out as described by 1993, OECD and Standard Methods for the Examination of Water and Wastewater, 1971. Assessment of mortality (quantal response) determined at the end of the 96th hour as a result of toxicity of zinc and copper on the test animals (Clarias gariepinus) was determined by the use of Finney’s Probit Analysis LC50 Determination Method [53].
Results
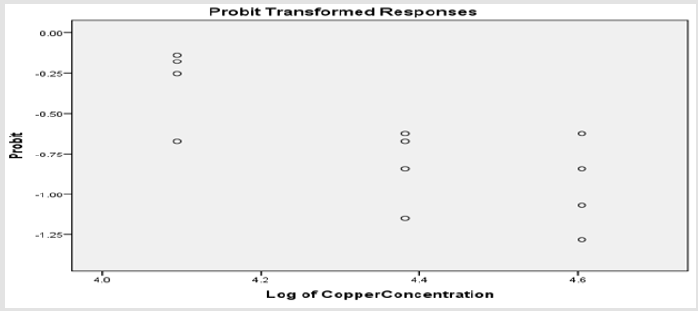
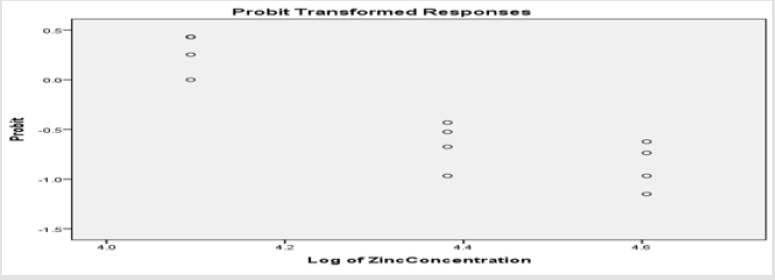
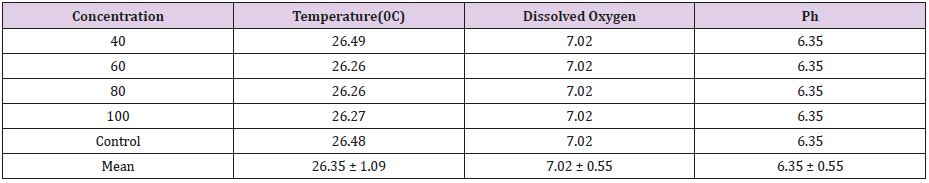
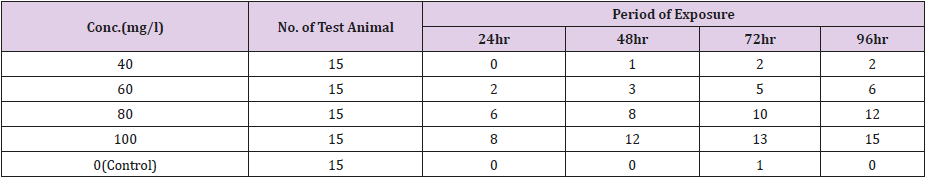
Table 1 shows the physio-chemical characteristic variables maintained in the aquaria for the toxicity testing of the fish species, while Tables 2 & 5 gives the relationship between the different exposure concentrations and the mortality response rate of Clarias gariepinus. The Probit parametric estimates and the obtained results for the acute static 96-hour toxicity estimated lethal concentration values and their confidence limits are shown in Tables 3,4,6 & 7 respectively. Figure 1 & 2 displays the probit line graph of acute toxicity of copper and zinc respectively for Clarias gariepinus and Table 7 showing the 96hrs acute toxicity of copper and Zinc on Clarias gariepinus.
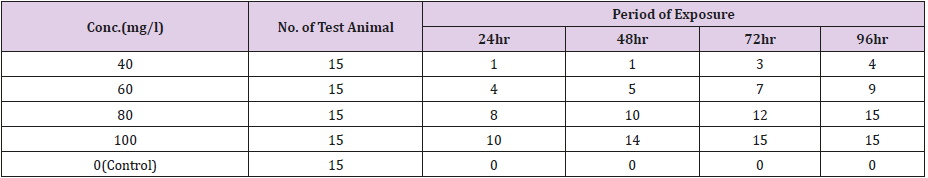
Table 2: The relationship between the zinc concentration and the mortality rate of Clarias gariepinus [54] for the 96-hour exposure.
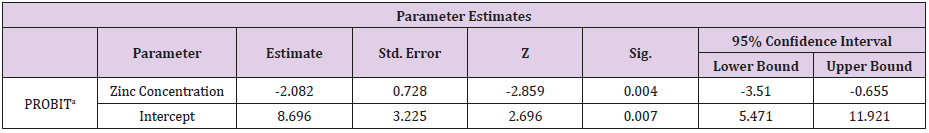
Table 3: Parameter Estimates for the Probit Analysis.
a. PROBIT model: PROBIT (p) = Intercept + BX (Covariates X are transformed using the base 2.718 logarithm).
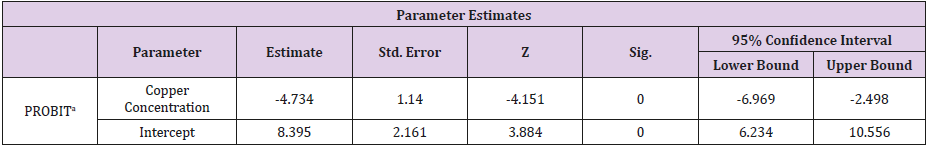
Table 4: The relationship between the copper concentration and the mortality rate of Clarias gariepinus [54] for the 96-hour exposure.
Table 5: Parameter Estimates for the Probit Analysis.
a. PROBIT model: PROBIT (p) = Intercept + BX (Covariates X are transformed using the base 10.000 logarithm).
Table 7: The relationship between the 1/10 zinc and copper concentration and the mortality rate of Clarias gariepinus [54] for the 96- hour exposure.
Physical Parameters of Well Water in the Aquaria Used for the Study
From the results of the Physio-chemical parameters measured and presented in table above, water temperature was recorded to be 26.35 ± 1.09oC; the dissolved oxygen concentration was 7.02 ± 0.55mg/l, while the ph was 6.35 ± 0.55 (Table 1).
The Relationship Between the Zinc Concentration and the Mortality Rate of Clarias Gariepinus for the 96-Hour Exposure
(Tables 2 & 3).
The Relationship Between the Copper Concentration and the Mortality Rate of Clarias Gariepinus for the 96- Hour Exposure
(Tables 4-6) (Figures 1 & 2).
The Relationship Between the 1/10 Zinc and Copper Concentration and the Mortality Rate of Clarias Gariepinus for the 96-Hour Exposure
(Table 7).
Discussion
The Finney’s probit analysis method was used to investigate 96-hour LC50 value for Clarias gariepinus exposed to different concentrations (40, 60, 80 and 100mg) for copper and Zinc respectively. Control mortality was however at 5%. 95% lower and upper confidence limits for the LC50 values were 44.394 and 67.848 mg/l for copper, while 36.3 and 75.36mg/l respectively were for zinc. These toxic effects of copper and zinc increased, (Table 2) (Figure 2) for zinc and (Table 4) (Figure 1) for copper as their concentrations were increased. Several factors such as the type of the tested heavy metal, solubility of the chemical characteristics of the test solution and the mechanism of action of the different metals, contributed to the different toxicity observed in tested metals which is also found to concur with the findings of [54]. All these factors determined the availability and penetrability of the metals into the test animals and hence, their toxicity. While working on the toxicity testing of Poecilia reticulatata in hard water (260mg CaCO3/L) [55] reported the 96-h LC50 value to be 55mg/L. Although this result is slightly lower to our observations in Clarias gariepinus (59.357 for copper and 65.151mg/L for zinc-Table 7). The difference could be attributed to variations physio-chemical properties (Table 1), which had already been documented.
From the study, it was observed that the test organisms became irritable over a period of time, but more erratic display were observed with increased concentration of the metals. The fish were seen swimming to the surface frequently with their opercula and mouths moving rapidly an indication that the Toxic effect of the heavy metals caused the depletion of the oxygen content of the medium. swimming Activity and frequent surfacing reduced drastically, color change from black to pale with mucus covering the body were observed. The mucus covering the entire body of the test organisms might have resulted from response to the toxic effect of the heavy metals through excretion of some accumulated metals in their tissues. Khalaf et al. [56] & Heath [30], supported this observation that the skin is an important excretory organ for heavy metals. Consequently, the age of the fish could also influence its response to a given contaminant or toxicant in a given medium as reported by [57]. Findings from this study on binary combination of the heavy metals is consistent with the observations of Hilmy et al. [30] who reported that copper was significantly more toxic than zinc for the 96hrs LC50 exposure with Clarias lazera [58-67].
Conclusion
From the present study, it can be stated conclusively that fish has the tendency to accumulate heavy metals in a polluted environment. Lethal effects of zinc and copper have been widely reported for different aquatic organisms and their exposure methods. Since these metals are an important constituent of industrial wastes discharged into water bodies, there is need to regulate the use of this heavy metal (zinc and copper) to prevent future damages in aquatic environment. Further study of toxicity testing methods on fish both in laboratory and in its immediate natural environment will be very useful in assessing possible ecological risk of heavy metals.
For more Articles: https://biomedres01.blogspot.com/





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.