Contemporary Approaches to Diagnostics and Treatment of Foramen Magnum Meningiomas
Introduction
There has been a significant change in the structure of intracranial tumors this past decade. According to CBTRUS, in 1994, gliomas totaled 46.5% (including glioblastomas - 23.3%) of all brain neoplasms, meningiomas - 24.8%, other tumors - 28.7%, in 2007-2011 the share of gliomas decreased to 27.8% (including glioblastomas - 15.6%), but the meningiomas percentage increased up to 35.8% and other tumors to 36.4% [1, 2]. Intracranial meningiomas, in the overwhelming majority, belong to the benign neoplasms (WHO Grade I-II) [3-5] with slow growth and progredient progression. A separate group consists of skull base meningiomas characterized by complex anatomical interrelations and high sensitivity of surrounding structures to surgical manipulations. The neoplasm removal is associated with solving the function preservation problem of intracranial neural and vascular structures, which is ensured using wide possibilities of microneurosurgery: surgical optics, neurophysiological monitoring, etc. [6]. Due to specific symptoms, complicated surgical anatomy and unique operating conditions, a special group of meningiomas is distinguished - meningiomas of the craniocervical junction or the Foramen Magnum (FM).
These neoplasms represent, by various estimates, around 0.3% to 1.8-3.2% of all intracranial meningiomas (1 case per 2-7 million people per year) [6,7]. It is believed that among all meningiomas, FM localization has the worst results in terms of postoperative mortality and surgical complications. The rarity of clinical observations, complex anatomical localization and high surgical risks suggest individual treatment planning. Anatomically, the region of Foramen Magnum is considered in the following borders: anterior - from the lower third of the clivus to the upper edge of C2 vertebra body; lateral - from the jugular tubercle of the occipital bone to the upper edge of C2 vertebra arc; posterior - from the anterior margin of the occipital bone scales to C2 vertebra spinous process [8-13].
Materials and Methods
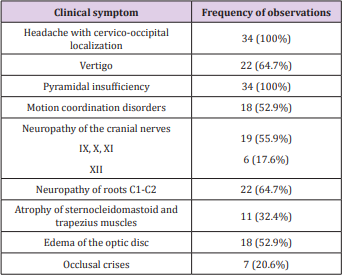
This study includes 34 observations; patients aged 18 to 75 years (median age 52.8 years) in the period from 1991 to 2016. There were 7 men, 27 women (ratio M: F -1:3.9). This amounted to about 1.6% of the total patient number with symptomatic meningiomas. It is believed that only 25% of meningiomas are symptomatic [9], and the rest are identified as incidental findings in routine examinations or autopsy. In our study, all tumors were symptomatic; there was not a single incidental detection of FM Meningioma on Magnetic resonance imaging (MRI) or CT. The duration of manifestation prior to diagnosis was 2.5 to 48 months (median duration 14.8 months). Clinical symptoms are presented in Table 1.
The most frequent disease manifestation symptom was pain in cervico-occipital area, which was enhanced by head flexion - was observed in all 34 (100%) patients. All of them were registered progressive pain in this specific location with growth to paresthesias in hands. These symptoms were the main and most distinct in the clinical manifestation of FM meningiomas. Pyramidal insufficiency of varying severity degree was also noted in all 34 (100%) patients. However, in 1 (2.9%) case the patient could not move independently for about 3 months because of tetraparesis, and in 4 (11.8%) observations there were significant motor functions limitations. Most of the examined patients had pyramidal disorders with moderate expression. 22 (64.7%) patients demonstrated vertigo. Somewhat less often, there were coordination disorders - 18 (52.9%) patients. Very frequent symptoms were the neuropathies of cranial nerves: IX, X, XI - in 19 (55.9%) patients, XII - in 6 (17.6%). Due to the accessory nerve lesion, the atrophy of trapezius and sternocleidomastoid muscles developed in 11 (32.4%) observations. 22 (64.7%) patients were registered thermal dysesthesia, astereognosis and anesthesia in C1-C2 dermatomes.
As a rule, patients made account of pain in the cervico-occipital location, motor disorders and ataxia. Occlusive crises were registered in 7 (20.6%) patients. Optic disc edema was detected in 18 (52.9%) examined patients. All patients, in the study and control groups, underwent a comprehensive examination according to neurooncological standards. The basis for FM meningioma diagnosis was CT and MRI integrated use. The main tasks of neuroimaging are the following: tumor localization in FM, rectification of its relationship with the brain stem (lateralization) and vertebral arteries, determination of extradural expansion. Meningiomas of small and medium sizes (up to ½ of FM diameter, less than 2.5 cm in the largest measurement) were observed in 8 (23.5%) patients, and large meningiomas (more than ½ of FM diameter, more than 2.5 cm in the largest measurement) - in 26 (76.5%). The lateral and anterolateral tumor location was observed in 31 cases (91.2%), posterior - in 2 (5.9%), anterior without lateralization - in 1 (2.9%) (Figure 1).
Figure 1: Foramen magnum meningiomas of ventral localization (arrows) with (left) and without lateralization (right).
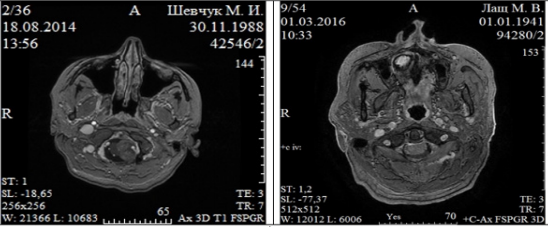
FM meningiomas at CT are determined as neoplasms of round or oval form of iso- or slightly hyperintensive density. Hypodensive meningiomas were not identified. The accumulation of contrast in all cases was mostly homogeneous. Intra-tumor cysts were not detected in any observation in FM meningiomas. Small size calcinates were detected only in 2 (5.9%) cases. IV ventricle was compressed and displaced or enlarged with caudal arrangement of the tumor. Hydrocephalic dilatation of lateral and third ventricles was noted in 25 (73.5%) observations. The wide tumor base adjacent to the dura mater was an indispensable feature of FM meningiomas. An invasive growth into the bone tissue was observed in 4 (11.8%) cases. Bone changes (hyperostosis) caused by FM meningiomas did not have a significant size in our study. These neoplasms were not characterized by perifocal changes, which are not very typical for meningiomas. The frequency of such changes caused by meningiomas with different localizations is 50-83% [6]. The possibilities of dual-energy SCT method allowed to not only provide 3D bone reconstruction with the tumor node projection on them, but also SCT - angiography with the projection of the main vessels onto the tumoral nodes (Figure 2).
Figure 2: Dual-energy spiral computed tomography of patient K., 55 y.o. Foramen magnum meningioma of anterolateral localization (left). Spiral computed tomography with intravenous contrast enhancement (up). CT angiography (middle). 3D reconstruction: tumor is painted blue, vertebral and basilar arteries dislocated by tumor to the right (bottom).
The internal structure heterogeneity of meningiomas and the wide variety of their MRI variants are determined by the histobiological tumor properties. No specific MRI patterns were traced by us in FM meningiomas. Practically all meningiomas were characterized by a pronounced homogeneous accumulation of paramagnetic intravenous contrast agent. Local perifocal changes around FM meningiomas were detected only in 5 (14.7%) patients, generalized perifocal changes - not detected in any case. One of the most significant FM meningiomas MRI signs is the cerebral fluid fissure between the tumor node surface and brain structures with blood vessels in it [6]. In FM meningiomas, unlike other meningiomas localizations, this specific sign was not detected in all cases. Full or partial obturation is caused by the arachnoid sheath adhesions with the underlying superficial parts of the brain, which are caused by proliferative changes developed at the tumor edge.
Complete preservation of the peritumorous cerebral fluid fissure was noted in 4 (11.8%) patients, partial obturation in 22 (64.7%), complete obturation in 8 (23.5%) (complete obturation is rarely observed with supratentorial meningiomas). Dura mater invasion was detected without contrast enhancement due to thickening of the latter and the outer contour deformation [6,7]. The use of contrast enhancement increased the abilities to diagnose dura mater neoplasm invasion. This symptom was noted in 7 (20.6%) patients. According to MRI data, tumor growth all around vertebral artery was detected in 12 (35.3%) cases (Figure 3). But this is not completely correct data, intraoperatively the dura mater invasion around the vertebral artery was observed in 18 (52.9%) observations.
We used the midline posterior (suboccipital) in 27 (79.4%) and posterolateral transcondylar 7 (20.6%) surgical approaches with lateralization. The midline posterior approach is considered optimal for posterior meningiomas - in these cases the brain structures are shifted anteriorly from the tumor. Posterolateral (far lateral or lateral) approach was first proposed by Koos WTH et al. [13], and Babu RP et al. [14], and modified by Heros RC [15] and Perneczk A [16]. It provides a lower-lateral approach, if necessary - double-sided, to the ventral surface of the brainstem without brain retraction. The described variants differ only in the condyles resection degree and mobilization of the vertebral artery. After the VA mobilization, it was displaced downwards, and the resection of the occipital condyle posterior part was performed with the drill. We avoided the junction resection more than 50% to avoid craniocervical instability. It is believed that partial condylectomy can significantly expand the surgical corridor.
The performed incisions of dura mater were Y-shaped or horseshoe-shaped, retracted with stitches. According to matrix accessibility, tumor resection was started with the devascularization of its posteroinferior portion. Then meningioma was enucleated in parts and separated from the rootlets of the spinal cord and lower cranial nerves. The medial and anterior tumor parts were removed last, after complete devascularization took place, in a dry operative field, with a minimal brainstem displacement. If the matrix was inaccessible, the removal was started with tumor enucleation using ultrasonic aspirator within visible limits with thorough gradual hemostasis. The enucleation area was expanded toward the matrix, which was coagulated, starting with its middle part. Gradually, the posterior tumor portion was devascularized and removed, releasing lower cranial nerves, cervical rootlets. Thus, surgical corridor was expanded significantly, which provided well-controlled manipulations on the remaining medial tumor portion and its matrix. After resection of the latter and cutting it off from dura mater the medial portion of the tumor was gradually dislocated and removed in parts. In 3 (11.3%) cases, the control of manipulations was provided by using an assisting endoscopy.
According to the dural insertion with the tumorous tissue, a partial or complete resection of its affected areas (anterior or posterior to the vertebral artery) was performed - 7 (20.6%) cases. Vertebral arteries mobilization from the tumor mass is associated with a risk of ischemia; therefore, it required thorough intraoperative neurophysiological monitoring. We succeeded in VAs mobilizing and dissecting them from the tumor all the way from the site of its penetration through the dura mater in 14 (41.2%) patients. In 4 (11.8%) cases, the VA mobilization and tumor resection were limited by neuromonitoring indications (a sharp decrease of somatosensory evoked potentials amplitude) or anesthesiological limitations (persistent bradycardia and blood pressure decrease). Muscle patch was used to close dura mater. Muscular and aponeurosis layers were tightly closed.
Results
To assess the extent of surgical treatment, we used the gradation system Simpson [17]. Total removal of FM meningiomas (Simpson 2) was performed in 24 (70.6%) cases, subtotal (Simpson 3) - in 6 (17.6%), partial (Simpson 4) - in 4 (11.8%). There was no postoperative mortality. Tumor resection was performed in two stages, in one case, because of decrease in blood pressure to 60 mm Hg and bradycardia during the first intervention, probably due to brainstem dislocation. The remaining tumor portion was completely removed after 5 months. In the early postoperative period, lower cranial nerves and C1-C2 rootlets dysfunctions were registered in 19 (55.9%) patients, and 13 (38.2%) had mild or moderate pyramidal disorders. Cerebellar ataxia was noted in 22 (64.7%) observations. Clinically significant pneumocephaly was noted in 3 (8.8%) cases, which required air extraction (patients were operated in the sitting position).
There were no hemorrhagic complications in any case. Postoperative radiosurgery (gamma-knife) was carried out in 1 (2.9%) case (after subtotal tumor removal), radiotherapy - in 4 (11.8%) cases after partial and subtotal FM meningioma resection. Functional outcomes were initially estimated according to the Karnofsky Performance Scale in the period from 6 to 14 months after the surgery. 90-100% (no disability or vital activity) - 23 (67.7%) patients, 70-80% (mild disability and vital dysfunction) - 8 (23.5%) patients, 50-60% (moderate disability and life-support needs) - 3 (8.8%) patients whose tumor was partially removed. There were no gross neurological disorders, which significantly restricted the ability to work and live. All patients were or continued to be monitored, according to clinical protocols. They were recommended to undergo a control MRI at least once a year. Catamnesis was up to 19 years. There were no repeated complaints about recurrence or continued FM meningioma growth in either case, even after subtotal or partial tumor removal.
Bulbar disorders and deep right-sided hemiparesis were observed in postoperative period in a single patient after separation of tumor from brain stem and vertebral artery (total tumor resection). Autologous MSCs from adipose tissue (6.0- 8.010^6 cells) were used in complex postoperative treatment (submucous perineural implantation [18-21]). Migration of MSCs led to distinct progressive recovery of functions: bulbar disorders completely regressed in four months, hemiparesis disappeared, and motor coordination recovered. Patient wears heels and likes dancing 4 months later. Information on the ability of stem cells to differentiate in a neuron-like direction after microglia activation is the fundamental basis for this tactic [22]. There are experimental data [23] confirming that implanted stem cells form synaptic contacts with endogenous brain neurons close to neurodestruction area after differentiation into neuron-like elements. By the way, it is paradoxical, but stem cells have antitumor effect also [24].
Discussion
Yasargil M et al. [11] analyzed all 114 cases of FM meningiomas surgical treatment, described in the literature from 1924 to 1976 and have set an average level of surgical mortality as 13%. By now, the largest groups of patients were represented by George B, Lot G [25] - a cooperative study of 143 craniocervical meningiomas in 21 clinics and Wu Z et al. [26] - analysis of 114 observations for over 15 years from one clinic (Beijing Tiantan Hospital). Bruneau M., George B. (2008) [9] provided a detailed review of 343 FM meningiomas cases described in English literature from 1987 to 2006, including 40 of their own observations. Pamir MN, Özduman K [27] in their review provided information on 467 surgically treated FM meningiomas, including 22 of their own observations, in the period from 1978 to 2006 (microsurgical period) and published information on three new own cases. Bruneau M, George B [8] described 57 new cases in addition to the 40 presented earlier in their publication. In addition, a number of articles have been published in recent years, in which 212 new cases of surgically treated FM meningiomas were presented [3,28,31-36]. Thus, about 750 cases of surgically treated FM meningiomas were presented in the accessible literature by 2016.
In the 1980s and 1990s publications, postoperative mortality was 0% [37], but in other reports it was 7.5% - George B et al. [38], Sen CN & Sekhar LN [39] - 20%, Kratimenos GP, Crockard HA [4] - 29%, Crockard HA, Sen CN [40] - 66%. Recent years studies show a decrease in postoperative mortality to 0-3.6% [27,28]. Bruneau M, George B [9] reported in their review that, before 2008, postoperative mortality ranged from 0 to 25% (mean 6.2%), and higher than 10% was, mainly, in small groups [41-46]. In 2004- 2015 works postoperative mortality in groups of 11 to 30 patients was from 0% [3,43,47] to 4.5-6.7% [36,44]. According to the largest analysis, by Wu Z et al. [26], which totals 114 observations - postoperative mortality was 1.8%. Our experience in diagnosing symptomatic FM meningiomas allows us to conclude that these neoplasms have a very specific clinical picture, and their most characteristic manifestations are typical pains in the cervicooccipital area, pyramidal disorders and lower cranial nerves and C1-C2 rootlets dysfunctions. The variability of the clinical symptomatology pointed out by neurologists of previous years [28], is not characteristic of these tumors. The FM meningiomas localization is perhaps the most important characteristic of these neoplasms, providing planning for the forthcoming surgical intervention. Traditionally, FM meningiomas are regarded as anterior, if their matrix is located ventrally on either side of the midline; lateral, if the matrix is located between the middle line and the dentate ligament; posterior, if the matrix is located posteriorly from the dentate ligament. Most meningiomas have anterolateral location (68-98%), less often they are localized in the posterolateral regions, even more rarely in the posterior ones. In most studies, anterolateral and anterior meningiomas are grouped together and considered as anterior or ventral [48-51].
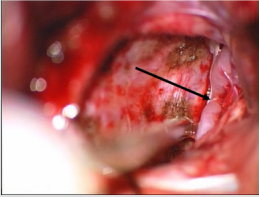
In our opinion, it is also necessary to mark their lateralization, for a more complete characterization of ventral meningiomas. We mean that the absolute majority of ventral FM meningiomas are lateralized, which determine the side of surgical approach (Figure 2). For example, the state after resection of Foramen Magnum meningioma of anterolateral localization (Figure 4). In our study, tumors of ventral location were lateralized in 31 (91.2%) observations and only in 1 (2.9%) case - ventral FM meningioma had no lateralization. Since surgical interventions for such neoplasms have their own specific characteristics, we believe that it is more appropriate to concentrate on the notion of a “surgical corridor”, rather than on ventral tumor location [2,9,13,25].
Figure 4: The state after resection of foramen magnum meningioma of anterolateral localization. Pieces of coagulated dura mater, brain stem and merge of vertebral arteries (arrow) with branching left anterior spinal artery can be found.
FM meningiomas are limited tumors that spread within the anatomical area without significant bone invasion, the removal of which usually does not require modified surgical approaches. Mainly ventral neoplasms location suggests the use of anterior transoral approaches. However, they proved to be ineffective due to significant drawbacks: increased risk of CSF fistula and infectious complications, poor access to laterally extending tumors resulting in incomplete resection, the risk of postoperative craniocervical instability and soft palate paralysis [12,26]. Currently surgical approaches are used for FM meningiomas resection, in accordance to the need of lateral surgical corridor extension: midline posterior, posterolateral transcondylar and ultra-lateral transcondylar. The most optimal for posterior FM meningiomas resection (posterior to the dentate ligament and medial to the vertebral artery) is the midline posterior approach [2,9].
The surgery is performed in patient’s “sitting” position (preferable position, to reduce venous bleeding) or “on the side” with rigid head fixation. The risk of venous air embolism is easily solved by anesthetic measures. Skull trepanation (usually resection) is performed in the lower part of the occipital bone with partial resection of C1 posterior arch. Dura mater is incised T- or Y-shaped, retracted with stitches. Goel A et al. [34] believe that even ventral FM meningiomas can be removed using this approach. With posterolateral access, there is a risk of vertebral artery damage at the condylotomy stage, or stricture formation due to excessive coagulation. Expanding the approach to the jugular tubercle is dangerous due to a high risk of lower cranial nerves damage [2,7,9,13,16,27,32,33,35,49].
It is believed that anterolateral (extreme-lateral) access (anterolateral approach or extreme-lateral approach) is indicated by the extradural extension of ventral and ventrolateral FM meningiomas. It was first described by Sen CN, Sekhar LN [39], modified by Salas E et al. [49] and Rhoton AL Jr [32]. It is fundamentally different from posterolateral suboccipital transcondylar approach by pathway. The patient head is laid with a turn of up to 60°. The skin incision is performed behind the mastoid process with the transition to the neck. The occipital bone, C1 posterior arch and lateral mass are allocated. The vertebral artery is mobilized up to the transverse process of C1, and then the transverse process is resected to the arterial canal. The venous bleeding is easily stopped by coagulation and the use of Surgical. The small branches of V3 segment are coagulated and intersected.
This ensures a safe resection of atlanto-occipital joint and the jugular tubercle. Often, the periosteum around the vertebral artery is calcified and requires careful manipulation. Retrosigmoid craniotomy is performed down to FM and the sigmoid sinus is exposed to the jugular vein bulb. If necessary, the VA transposition [47] is performed, especially with extradural meningioma expansion. C1 and C2 laminectomy is performed to the lower pole of the tumor. After this, the extradural component and the altered bone structures of FM anterior part can be removed. Tumor invasion in this case indicates the volume of bone resection to ensure maximum tumor resection. Then the dura mater is incised, and tumor resection begins [2,3,5,7,13,17,27,3s8,49].
The use of intraoperative neurophysiological monitoring of somatosensory evoked potentials, short latency auditory evoked potentials and electroneuromyography of lower cranial nerves by recording through the intubation tube (CN X) and with the needle in the sternocleidomastoid muscle (CN XI) and the tongue (CN XII), is considered appropriate [9,13]. Bassiouni H et al. [41], all surgical approaches are divided into two types-retrocondylar, when the articular processes are not resected, and transcondylar, when condyle resection of any volume is performed. In recent years, advantages and disadvantages of these methods have been critically evaluated, and many specialists have begun to use less laborintensive posterior suboccipital access to remove ventrolateral and even ventral FM meningiomas [34].
The level of transitory postoperative complications ranges from 39.3%, permanent - from 7.1%. Usually these are lower cranial nerves dysfunctions and ischemic problems are associated with the vertebral artery. The surgical medulla oblongata trauma of neuraxis is rare. The sublingual nerve and jugular vein are most often traumatized during occipital condyle resection [3,36,39,40]. Conclusion. Suboccipital lateralized approach with laminectomy to the lower tumor pole is enough to provide adequate microsurgical FM meningiomas removal without atlanto-occipital junction resection. Matrix approach should be performed after partial tumor resection without brain stem traction. The use of intraoperative neuromonitoring provides stem functions control at all stages of tumor removal and spinal artery mobilization.
Conflict of Interest
All listed authors concur with the submission of the manuscript; all authors have approved the final version. The authors have no financial or personal conflicts of interest.
For more Articles: https://biomedres01.blogspot.com/





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.