Evaluation of Iron Toxicity in the Tropical Fish Leporinus frideric
Introduction
Toxicity is the inherent and potential capacity of the toxic agent to cause harmful effects on living organisms Borges [1]. The toxic effect is generally proportional to the concentration of the toxic agent at the site of action Klüver et al. [2].
Acute toxicity is characterized by the administration or exposure of the chemical in a single or multiple dose in a short time (hours), analyzing the adverse effects occurring within that time interval Albinati et al. [3]. The effects are due to a single contact (single dose) or multiple contacts (cumulative effects), arising immediately or over the course of a few hours or days Oga [4]. The acute toxicity test estimates the median lethal concentration (LC50) and classifies hazardous substances intoxicants and allows the establishment of permissible limits for various chemical substances, as well as assessing the impact of mixtures of pollutants on aquatic organisms of water bodies by tests simulating the natural conditions in the laboratory Qu et al. [5]. Metals from mining may be responsible for contamination of aquatic environments. Some metals, such as iron, zinc, magnesium, cobalt, are useful in small amounts as they form some cell structures. But if the limiting amount of these metals is exceeded, they will become toxic, causing problems to organisms exposed to these metals Teixeira & Bessa [6].
The element iron is considered a heavy metal being found in high concentrations in aquatic environments near the miners that exploit this ore. In Brazil, the dam located in the municipality of Mariana - MG was built to serve as a deposit of the wastes generated during the mining process of iron, and ruptured, causing an unprecedented environmental disaster in Brazil’s history Barba [7]. Iron is also regarded as an important chemical element for many living organisms (as a biometal) and is vital for survival at low concentrations as it is essential for multiple metabolic processes such as oxygen transport, DNA synthesis, electron transport, and cofactor for many proteins Bury [8]. Iron present in water can be absorbed by the fish via gills, skin or food Cottet et al. [9].
Excess iron dissolved in the water can cause the formation of flakes of this metal in the gills of the fish resulting in its obstruction, causing respiratory disorders (Zahedi 2014). Animals that consume diets with high levels of iron may have reduced growth, worse feed conversion, diet rejection, mortality, and histopathological damage in liver cells, where excess of iron in the body is stored Bury [8]. Fish can be used as environmental indicators, reacting immediately to any changes in the aquatic ecosystem, through physiological changes such as changes in opercular beating, unusual swimming, gill changes and feeding changes or difficulties Hundley et al. [10]. Such reactions are easily noticeable due to chemical intoxication Niencheski et al. [11]. The observable effects in the face of poisoning are various. Opercular beats are responses to changes in the aquatic environment, such as contamination by substances with toxic potential Gibson & Mathis [12]. Blood also has elements that respond to exposure to pollutants.
The same is responsible for the transport of substances in vertebrates, this tissue being directly linked to physiological dynamics and the body’s immune responses. The study of its cellular components, especially the study of glutathione (GSH), hemoglobin (Hb) and methaemoglobin (MeHb), can provide important information about stress and innate immunity of fish Maciel et al. [13]. Some metal ions (especially copper, zinc and iron) can bind to proteins involved in neurodegeneration. It is known that iron is responsible for several neurodegenerative pathologies in humans such as Alzheimer’s and Parkinson’s disease Koslowski et al. [14,15]. These diseases are triggered by genetic predisposition, aging and by environmental factors such as exposure to heavy metals Tan et al. [16]. Fish are important sources of dietary iron for the human organism, contributing to the formation of hemoglobin; however, over accumulation of iron ions in the human body by ingestion of foods with excess of this element may promote neurodegeneration Wojtunik-Kulesza et al. [17]. In this way, the aquaculture activity carried out by riverine communities in water bodies affected by iron pollution can be alarming.
The “piau” Leporinus friderici (Characiformes, Anostomidae) is one of the most widely distributed species in the Neotropical region. In addition, it is considered to be one of the most abundant species in the various water systems of the Amazon region with relevant economic importance Olivatti et al. [18]. The species of the genus Leporinus present great potential for fish farming because they have good commercial acceptance Baldisserotto & Gomes et al. [19]. Sexual maturation occurs when the individual reaches approximately 20 cm in total length, concentrating his reproductive period in the months of November to February. The “piau” performs seasonal reproductive migration (piracema). There is demand for L. friderici fingerlings for aquaculture and repopulation programs in rivers and reservoirs in much of the national territory Suplicy [20]. It is important to consider the risk of contamination of fish such as the “piau” with iron ions and the possibility of bioaccumulation and transfer to the human body through feeding. The present study aimed to determine the toxicity of iron ions (Fe2+ and Fe3+) in “piau” (L. friderici) juveniles by means of acute toxicity tests, opercular beating and blood parameters
Material and Methods
The experiments were carried out in the ichthyology laboratory of the Institute of Scientific and Technological Research of the State of Amapá - IEPA. Eighty eight specimens of “piau” (L. friderici) with an average weight of 1.70 ± 0.68 g were used. All animals were measured with digital caliper QS-50 (Jakemy, China), weighed in precision digital scale 0.01 g BL series (Shimadzu do Brasil Comércio Limitada, Barueri, SP, Brazil). The fish were purchased at a fisherman’s shop, acclimated for a week and fed daily (08:00 and 17:00 h). Water with an average temperature of 27°C ± 1.0 and measured using the mercury thermometer (Accumed Produtos Médico Hospitalares Ltda., Duque de Caxias, RJ, Brazil). The pH presented an average value of 7.25 ± 0.75, using a HI 98129 digital parameter (Hanna Instruments Inc, Woonsocket Rhode Island, USA). Dissolved oxygen concentrations were always close to saturation.
Feeding was suspended 24 h before the start of the experiment, according to the recommendations of the Brazilian Association of Technical Standards - ABNT ABNT [21] for acute fish toxicity tests. The water employed in the experimental media was treated with AquaSafe (water chlorine remover) and parasite controller Labcon Ictio (Alcon) to avoid parasites in the animals. The treatments were carried out in 2 L beakers (one fish per beaker) with aeration (24 h) with the use of compressor pumps for aquariums (model U-2800, Boyu). The three “piau” specimens were divided into 11 groups and added iron in the water for the test with buffer solutions of ferrous sulphate and ferric sulphate and maintained for up to 96 h. The concentrations tested were: control (without addition of iron); 1 mg/L Fe2+ and Fe3+; 3 mg/L Fe2+ and Fe3+; 7.5 mg/L Fe2+ and Fe3+; 15 mg/L Fe2+ and Fe3+; 30 mg/L Fe2+ and Fe3+, the same tests were performed for each concentration, each fish being one replicate (n = 8). For the analysis of the effects caused by exposure to Fe, the opercular beats (OB) were observed. At this time, the specimens were kept individually in a beaker for one minute before and after submission to the treatments cited for the period of one hour where OB counts were performed per minute. Mortality was also assessed throughout the experiment. For the collection of blood, the animals were collected still dying, avoiding blood clotting.
To determine the GSH, 50 microliters of whole blood were collected from caudal venous puncture with the use of heparinized capillaries, diluted 60 times in 3 mL cuvette, for further reading in a spectrophotometer at 412 nm. The determination of hemoglobin was performed according to the methodology described by Clark et al. [22]. Blood samples were collected from the dying fish and analyzed immediately in a spectrophotometer at 540 nm. Methemoglobin levels (MeHb) were determined according to Benesch et al. [23], also with the use of capillaries with heparin, with subsequent reading made at 630 nm in a spectrophotometer. The data were submitted to non-parametric ANOVA by the Kruskal-Wallis test by the INFOSTAT version 2017 program Casanoves et al. [24]. Significant differences were considered when the value of p <0.05.
Results and Discussion
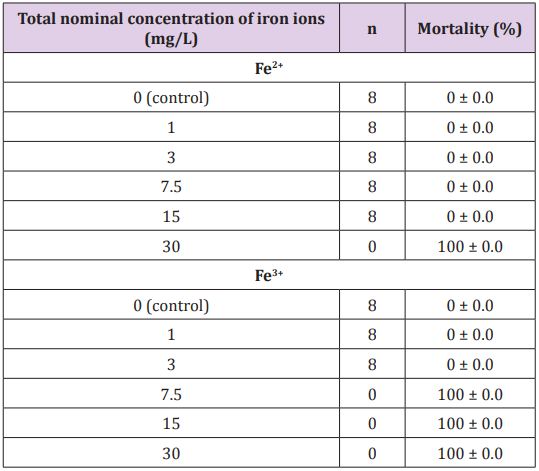
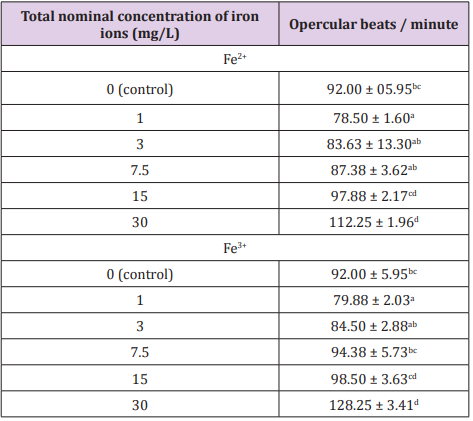
The results of the iron ion toxicity in juveniles of L. friderici indicated variability of the toxic effects. Fe3+ showed toxicity at lower concentrations and at shorter exposure times in comparison with Fe2+. Total lethality was observed after 3 h of exposure at concentrations of 7.5 mg/L; 15 mg/L and 30 mg/L Fe3+. Fe2+ ions showed lower toxicity, but the total lethality was observed in 24 h of exposure at 30 mg/L Fe2+ concentration. At concentrations of 1 mg/L; 3 mg/L; 7.5 mg/L and 15 mg/L Fe2+, no mortalities were observed after 96 h of exposure (Table 1). It was observed that for 30 mg/L Fe2+ (1h = 112.25 ± 1.96 OB/min, 24h = total lethality); 15 mg/L Fe2+ (1h = 97.88 ± 2.17 OB/min); 7.5 mg/L de íons Fe2+ (1h = 87.38 ± 3,62 OB/min; 96h = no lethality), 3 mg/L Fe2+ (1h = 83.63 ± 13.3 OB/min; 96h = no lethality); 1 mg/L Fe2+ (1h = 78.5 ± 1.60 OB/ min; 96h = no lethality).
Table 1: Mortality in “piau” juveniles after acute exposure to iron ions (Fe2+ and Fe3+) in water for 96 hours.
Tests that presented the highest lethality were those performed with Fe3+. It was observed that at 30 mg/L Fe3+ (1h = 128.25 ± 3.41 OB/min; 3h = total lethality); 15 mg/L Fe3+ (1h = 98.50 ± 3.63 OB/ min; 3h = total mortality); 7.5 mg/L Fe3+ (1h = 94.38 ± 5.73 OB/ min; 3h = total lethality); 3 mg/L Fe3+ (1h = 84.50 ± 2.88 BO/min; 96h = no lethality); 1 mg/L Fe3+ (1h = 79.88 ± 2.03 OB/min; 96h = no lethality). Iron is an element considered to be of low toxicity at concentrations below 3 mg/L and can cause harmful effects to fish in their soluble form (Fe2+ or Fe3+), as demonstrated by Geertz Hansen & Rasmussen [25]. According to these authors, iron soluble at concentrations higher than 0.5 mg/L caused a significant decrease in survival in trout larvae (Salmo trutta).
In the present study, lethality was lower when fish was exposed to Fe2+, 30 mg/L (24h = total lethality); 15 mg/L (24h = no lethality); 7.5 mg/L (96h = no lethality); 3 mg/L (96h = no lethality). Studies show that other species of fish are tolerant to these concentrations of iron ions, such as zebra fish Danio rerio. According to Chua et al. [26] D. rerio has accumulated iron in the liver when exposed to 50 mg/L, suggesting that this metal could be accumulated in this organ. Significant increases were observed when compared to the control group (92.00 ± 05.95 BO/min), to the groups intoxicated with 30 mg/L of Fe3+ and Fe2+ (p<0.05) in the first hour (while all the specimens were still alive)(Table 2).
Table 2: Opercular beating in “piau” juveniles after acute exposure to iron ions (Fe2+ and Fe3+) in the water during the first hour of testing.
Different letters in the same column showed significant differences p<0.05 by the Kruskal-Wallis test.
The increase in opercular beats was higher in the groups contaminated with Fe3+ ions, since this was the most toxic ion. With the increase of the concentrations to 7.5 mg/L, 15 mg/L and 30 mg/L Fe3+, the beats changed, because, with the increase of this ion in water, dissolved oxygen (DO) decreased, causing the animals to keep their swimming altered in search of oxygen, provoking a growing stress in the organism since the search for oxygen increases along with BO. The liver, gills and skin are the major sites of iron deposition and storage under conditions of overload, the liver metabolizes excess iron in the plasma and stores it in the form of ferritin and hemosiderin Chua et al. [26]. The variation in the number of opercular beats is related to the concentrations of Fe2+ and Fe3+ from which L. friderici was submitted.
At low concentrations 3 mg/L and 1 mg/L of the two ions, there was little oscillation in the beats, because they are concentrations in which the organism can survive and make sufficient withdrawal of DO from the water to supply its needs Watanabe et al. [27]. Bury et al. [28] concluded that the ferrous form (Fe3+) is more toxic than the ferric form (Fe2+) for European plaice (Platichtys flesus) and iron uptake occurred mostly in the final part of the intestine. The most important factors that may influence iron uptake are the proportions of the mineral in organic and inorganic form (in feed and water), the amount ingested and the physiological conditions of the digestive tract Watanabe et al. [27]. In fish, the initial site of accumulation of metals absorbed from water is the gill tissue Zahedi et al. According to NRC [29], one of the effects of Fe toxicity on fish is loss of growth.
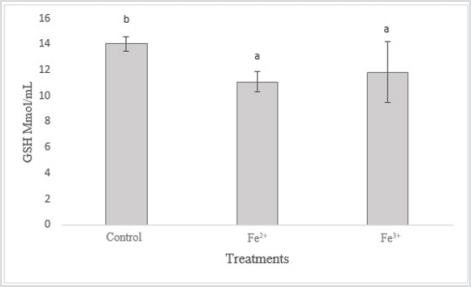
Glutathione levels decreased in the contaminated groups when compared to the control group (Figure 1). The greatest decrease in GSH occurred in the group contaminated with Fe2+, the time of exposure in these groups was higher, because it was where the greatest survival occurred during the 96 h of experiment, occurring only in the group of 30 mg/L Fe2+ in 24 h. In the groups contaminated with Fe3+, there was a lower decrease in GSH levels, since the exposure time was lower, that is, in less than 24 h there were total lethality in the groups of 30 to 3 mg/L of Fe3+
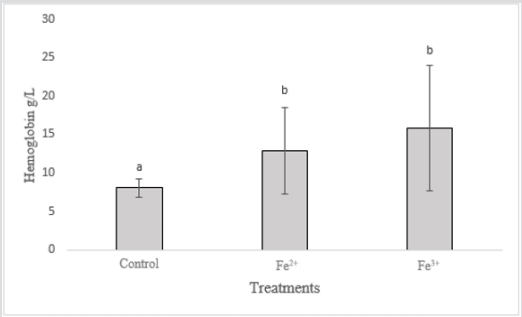
GSH can be considered one of the most important agents in the defense of cells against oxidative stress and plays a central role in the biotransformation and elimination of pollutants. This molecule has a reducing role in many reactions of peroxides and free radicals Huber et al. [30]. According to Van Der Oost et al. [31], the decrease in GSH concentration would indicate acute stress, affecting the immune system, the nervous system, and the gastrointestinal system. There was an increase in hemoglobin levels in the contaminated groups in relation to the control group (Figure 2). It is noted that increased hemoglobin may be associated with increased energy/oxygen demand by the body, which usually occurs in acute stress situations.
In the groups contaminated with Fe3+, the hemoglobin increase was more relevant, due to the toxicity of this contaminant being greater and causing damage to the organism in a shorter time. With the Fe2+ contaminated groups, there was also an increase, but smaller when compared to the Fe3+ treatments. Fish exposed to Fe ions have demonstrated a functional increase in blood hemoglobin levels, as Hb is converted to MeHb, causing intoxication in animals Aggergaard et al. [32]. Iron is involved in hepatic processes, and is also associated with oxygen transport through Hb, being considered one of the most important elements for fish homeostasis (Neves 2016). However, excess iron dissolved in the water can cause iron flakes to form in the gills of the fish resulting in their obstruction, causing respiratory disorders BURY et al. [8] and the increase of OB to overcome this provoked deficiency. In acute cases of iron poisoning there may be necrosis of the gill tissue and loss of ammonia excretion capacity that is concentrated in the blood of the fish Slaninova et al. [33].
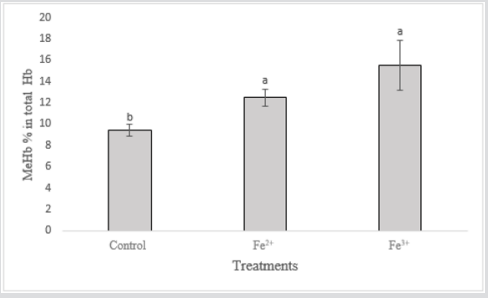
Methemoglobin levels increased in groups contaminated with iron ions (Figure 3). The increase of MeHb in the organisms exposed to Fe3+ was higher in relation to the control group and the fish exposed to Fe2+, the latter being also larger than the control. It has already been shown that Fe3+ are more lethal than Fe2+, thus toxicological effects are more conspicuous in the groups contaminated with this pollutant. The most important effect of iron on fish refers to the ability of this compound to oxidize Hb in the blood, converting it to MeHb Jensen [34], making it impossible to transport oxygen to tissues and possibly causing the death of the animal through asphyxia Avilez et al. [35]. In humans, the malfunctioning in the iron metabolism or its excess in the organism, generated oxidative stress and the production of oxygen radicals, being able to be related to Parkinson’s and Alzheimer’s disease Kozlowski et al. [15]. However, it is not known whether this accumulation is the cause or consequence of this disease Bury et al. [8]. Inflammatory problems of the central nervous system have been reported due to excess iron causing neuronal degradation Andersen et al. [36]. Therefore, it is advisable to avoid the consumption of fish contaminated with iron ions [37,38].
Conclusion
The two iron ions (Fe2+ and Fe3+) are toxic to the “piau”, and Fe2+ was toxic only at the concentration of 30 mg/L. Fe3+ causes total lethality at concentrations of 30 mg/L, 15 mg/L, 7.5 mg/L. At concentrations below 3 mg/L Fe3+, the organism tolerates acute exposure with no mortality. Therefore, Fe2+ is less toxic when compared to Fe3+, being tolerated by L. friderici at concentrations below 30 mg/L for 96 h of exposure. When compared to the control group, the opercular beats (OB) were higher with the concentrations of 30 mg/L with both iron ions. Considering the above, it is possible to observe that the decrease of GSH occurs in both groups, being higher in the group contaminated with Fe2+ ions, in these groups the survival time was higher, thus increasing the exposure to the pollutant. It is observed that the increase of hemoglobin was higher in the groups contaminated with Fe3+, since this one is more toxic. In the groups contaminated with Fe2+ also increased, but smaller when compared to the Fe3+ groups. Methaemoglobin increased in the Fe3+ groups, being higher in relation to the control group and the Fe2+ groups, and the latter being also larger than the control group.
For more Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.