Role of Nickel (II) Salicylhydroxamic Acid and its Aniline Adduct as an Antimicrobial Substitute
Introduction
Metal complexes which are formed in biological systems between a ligand and a metal ion are in dynamic equilibrium with the free metal ion in an aqueous environment [1]. All biologically important metal ions can form complexes and the number of different chemical species which can be coordinated with these metal ions is very large. During the past few decades, a lot of scientist research groups operated through specialization in the direction of drug discovery, by studying the simplest species that use metal ions and researching them as whole compound; for example, they suggested the addition of metal ion to antibiotics to facilitate their spread throughout the body [2]. The development of drug resistance as well as the appearance of undesirable side effects of certain antibiotics has led to the search of new antimicrobial agents with the goal to discover new chemical structures which overcome the above disadvantages. As a ligand with potential oxygen and nitrogen donors, hydroxamic acids are interesting and have gained special attention not only because of the structural chemistry of their coordination modes, but also because of their importance in medical chemistry [3].
Preparation of Salicylhydroxamic Acid (SHA)
Salicylhydroxamic acid was prepared by adding 19.0g of hydroxylamine hydrochloride to 200ml of 12% solution of caustic soda (NaOH, 24g) and cooled at room temperature. 35ml of methyl salicylate was then added in small quantity and vigorously stirred continuously to ensure complete dissolution. The mixture could stand over a period of fourteen days until the solution turned straw brown and was then acidified with 2 molar H2SO4 acid (60ml). The pale precipitate was then filtered, washed and re-crystallized from water containing little nitric acid. (Yield =20.25g).
Preparation of Nickel (II) Salicylhydroxamate Complex [Ni (Saha)2]
0.5g of nickel nitrate solution was added to 5g of salicylhydroxamic acid (SAHA), the solution was stirred to mix properly for complete precipitation. 0.18g of ethanol was added to the mixture. The precipitate was then filtered and dried over silica gel in desiccator (Yield=1.24g).
Preparation of the Adduct
0.4g of aniline in 25ml of mixture ethanol and water (in the ratio of 3:1) was added to ethanoic solution of Ni (SAHA)2 (1.20g), while stirring at 60oC followed by refluxing for 20minutes. A pale pinky precipitate was observed. The mixture was cooled to room temperature and the precipitate filtered and dried over anhydrous potassium hydroxide in a desiccator (Yield=0.65g).
UV Spectrophotometer Analysis
A UV spectrophotometer machine was used to determine the complexes and the anion made up. The wavelength was selected for each anion. The result in mg/L was displaced and recorded [4].
Solubility Studies
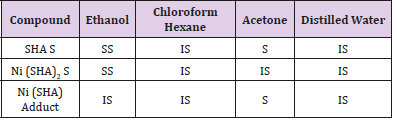
The solubility of salicylhydroxamic acid, Nickel (II) and its aniline adduct were dissolved in the following solvents; hexane, chloroform, acetone, water and ethanol at room temperature.
Conductivity Test
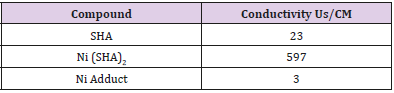
The conductivity test for the sample was carried out by first rinsing the electrode thoroughly with deionized water before carefully swiping with a tissue paper. Specific quantity of sample is measured and transferred into a beaker before placing it on a magnetic stirrer. The electrode is then dipped into the sample solution and held in place for a steady reading. Note and record the reading appropriately [5].
Antimicrobial Susceptibility Test
In vitro antimicrobial activity against selected bacterial and fungal agents including Streptococcuspyogenes, Shigelladysenteriae and Candida albicans were investigated by the disc diffusion method (Kasuga 2001). Mueller–Hinton agar (Difco, Detroit) was used for all bacterial strains, RPMI-1640 medium (Sigma-Aldrich) for the yeast strain. A total of 1mL of 1 × 106 spore/mL suspension was streaked on the respective agar plates for both bacterial and fungal agents. The plate could air dry in laminar flow before four sterilized Whatman® filter paper discs (diameter 6mm) were placed on it. A total of 20μL of the test compound was spread on the discs accordingly by using micropipette. Each test was conducted in triplicate [6]. The inoculated plate was then allowed to air dry and incubated under the standard incubation condition for seven days before the inhibition zone was recorded in percentage using the formula below.

Results, Discussion and Conclusion
Results
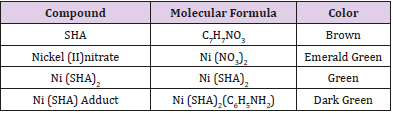
The results of the prepared compound and analysis are given below (Table 1):
Discussion
The synthesis of ligand and the nickel complexes and its aniline adduct were achieved with good yield. From the solubility test analysis carried out on the complex and the ligand, it shows that the compounds are soluble in polar solvents as shown in Table 2. The result is in line with previous studies as carried out by [7] who reported that SHA and its metal complexes were soluble in polar compounds. This solubility property observed in this study is responsible for the antimicrobial efficacy of SHA and its metal complexes. The solubility potential enhances their bioavailability and enables them to effectively permeate through various systemic membranes, thus facilitating their absorption and exertion of antimicrobial effects. The result of electrical conductivity as shown in Table 3, reveals the positive potential of the compound to be released commendably. This study confirms the appreciable conductivity and kinetics of release of the compound to play a major role in enhancing its antimicrobial effects.
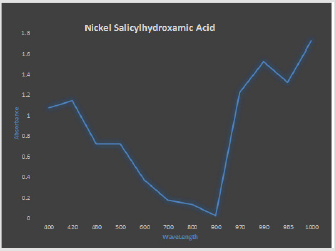
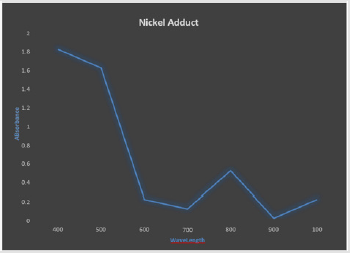
The UV spectrum for SHA, Ni(SHA)2, and Ni(SHA)2C6H5NH2as presented in Figures 1 & 2 respectively, are similar to previous results obtained from preceding studies which reported the electronic spectral of the Ni complexes and it adducts aniline complexes to be due to 3A2g-3T1 (P)transition of distorted octahedron. The result of absorbance at different concentrations exhibited by the compound as shown in Figures 1 & 2 is indicative that this compound has a preferred absorbance which is responsible for enhanced metabolism and bioavailability following absorption of the compound [8] reported the electronic spectrum of the nickel complex to shows two bands at 18900 cm-1and 13500 cm-1 which are attributed to 1A1g-1A2g and 1A1g-1B2g transitions. These transitions suggest a square-planar stereochemistry of the compound [8].
The result for in vitro antimicrobial activity against the selected bacterial and fungal organisms; Streptococcus pyogenes, Shigella dysenteriae and Candida albicans revealed that all the organisms were sensitive to salicylhydroxamic acid and Nickel salicylhydroxamate complex, but they were resistant to the aniline adduct. The results of this study are in line with previous studies as carried out by [7] who reported that SHA and its metal complexes possessed both antibacterial and antifungal antimicrobial properties. The mode of action may involve the formation of a hydrogen bond through the nitrogen and oxygen atoms in SHAM with the active centers of the cell constituents, resulting in interference with the normal cell process [7]. The resistance observed in nickel adduct may be due to limited number of representative microbial organisms or variation in species and strains of the representative micro-organisms evaluated in this study.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.