Expressions of Growth Factors in Autologous Derived Platelet-Rich Plasma and Platelet-Poor Plasma; Implication for Tissue Reparation and Wound Healing
Introduction
Growth factors (GFs) released from activated platelets initiate and modulate healing in both soft and hard tissues [1-5]. A recent therapeutic strategy to promote wound healing is through preparing autologous platelet concentrate (APC) suspended in plasma, known as platelet-rich plasma [6,7]. Platelet-rich plasma (PRP) acts as a storage vehicles for GFs, of such are PDGF [AA,AB and BB], TGF-beta 1 & 2, FGF- acid and basic, EGF, IGF and VEG [1,2,8,9] These GFs have been identified as a central player in the wound healing cascade and are known to influence the process of tissue regeneration [1]. Several publications have demonstrated that PRP has a great potential in the field of degenerative medicine and tissue reparation process [3,10]. However, despite the popularity of the use PRP, this novel technique is still plagued with several flaws of which includes inconsistence in the method of preparations and non- reproducibility of the GF expression when attempts are made to quantify their level of expression.
Some authors have alluded that the inconsistence and the difficulty encounter in ascertaining the quality of PRP preparation at a standardized pattern could be as a result of the followings; poor handling technique, the use of animal-derived thrombin for clotting, and fundamental individual idiosyncrasy [6]. To overcome these drawbacks, Anitua et al. developed plasma rich in growth factors (PRGF) by modifying the procedure of PRP preparation [7], has simplified the preparation protocol and replaced the animalderived thrombin with calcium for clotting. In addition, the Pointof-care devices system has helped to standardize the process of PRP preparation and production, which makes it useable at the bedside. This process involves fractionating the whole blood into PRP, platelet -poor plasma (PPP), PRP and red blood with a buffy layer of white cell coat [11], this is then activated by autologous derived thrombin to create a viscous gel known as PRP. Choukroun et al., demonstrated that the variability in the quality of the PRP gel is a function of the sequestration process and the speed of centrifugation, which he described as advanced plasma rich fibrin (soft fibrin clot) and the concentrated growth factor (stiffer fibrin clot) [12]. Therefore, it has been anticipated that the difference in mechanical characteristics may produce a difference in the growth factors expression [12]. However, it is unknown whether there is variability in the pattern expression of GFs from autologous derived PRP or PPP in diabetic patients and healthy volunteers. The purpose of this study was to compare the levels of growth factors released from PRP/PPP from diabetic patients with the chronic ulcers with healthy donors.
Material and Method
Study Design
Six male diabetic patients with chronic foot ulcers, age ranging from 45- 68 years as well as fourteen healthy volunteers (5 female and 9 male), age range of 18-40 years were recruited. Research Ethics approval was obtained from the East and City of London Research Ethic committee and informed consent was obtained from all volunteers and patients. In addition, the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in approval by the institution’s human research review committee. Platelet count from the whole blood and PRP in diabetic patients was analyzed but the platelet count was not measured in healthy donors. The rationale for measuring the Platelet count in the diabetic’s patient with other medical condition was to ascertain the platelet count was within normal range (100,000-300,000/µl), which was assumed will be appropriate to generate good quality PRP. In addition, this was used to validate the concentrating capacity of the point-of-care device used in this study (Angle Sorin). GFs level from PRP and PPP in both diabetic patients and healthy donors were measured. These was analyzed using the traditional ELISA technique for P-selectin and IGF, whilst the remaining GFs were measured using multiplex fluorescent immunoassay technique (MFI). MFI has a few advantages over ELISA in that it has increased sensitivity, specificity and better reproducibility. The GFs measured with ELISA were not available on the commercial multiplex kit. However, in a study conducted by research animal diagnostic laboratory (RADIL), they showed a correlation of greater than 99.5% between MFI ELISA.
Preparation of Platelet-Rich Plasma
The PRP was routinely processed in a sterile environment. Six-millimeter anticoagulant citrate dextrose -A (ACD-A) solution was drawn into 60-cc syringe, followed by 54 cubic centimeters of whole blood drawn from a large antecubital vein, the acid dextrose serves to preserve the integrity of the platelet membrane. The blood was gently agitated by mixing the anticoagulant with the blood. The 60 ml of blood was injected into the point of care centrifuge system (Angle, Sorin Group, Mirandola, Italy) and centrifuged for 25 minutes (first hard spin) at 5200rpm, followed by a soft spin at 3200rpm for another 45 minutes. The whole blood is sequestered into a semi-automatic controlled operation mode by centrifugation, through the whole blood separation processing system (Angel; Sorin), which separates the blood into PRP which contains platelet concentrate and leukocyte, PPP and erythrocytes. The desired product (PRP) is usually 10% of the starting volume of the whole.
PRP and PPP Activation
To initiate the release of the GFs from the PRP, the platelets must be activated, using the potent platelet activator thrombin. Human thrombin was generated from a commercial thermogenesis which consists of [66% volume for volume of Ethyl Alcohol, U.SP.25Mm Calcium Chloride U.S.P], stored at between 15-30°C , as a kit called “Activat” [Sorin Group, Mirandola Modena, Italy 41037]. Thrombin was generated after 12ml of PPP was mixed with the “Activat” beaded material in a pressurized syringe for about 20-25 minutes and the thrombin was squeezed out of the syringe, which generates a total of 4-6ml. Autologous thrombin is preferred in the United Kingdom to pre-prepared bovine thrombin, which has been implicated in the development of antibodies to clotting factor Va, XI and thrombin. The harvested PRP was combined with thrombin for platelet activation to produce the gelatinous material in ratio of 1:10 [volume/volume]. Similarly, the PPP was activated using the thrombin in the same ration. Following activation, the formed gelatinous material was left in a 15ml falcon tube overnight at 4°C, this allows for maximum clot retraction. The supernatant from the gelatinous coagulum was removed after centrifuging for 10 minutes at 3000rmp and is stored at -80°C, until GFs was ready for analysis. The supernatant was much easier to handle when performing the ELISA as compared with the gelatinous coagulum.
Hematology Analysis of Whole Blood and Platelet-Rich Plasma
Blood samples from diabetic patients (5ml of whole blood and 3-5ml of inactivated PRP) were retained in EDTA bottles. Each sample was counted in triplicate and then average (mean +/-SD). Complete blood count was carried out with the Beckman Coulter LH750 analyzer. This step was repeated for the platelet-rich samples.
Quantification of Growth factors in Platelet-Rich Plasma and Platelet-Poor Plasma
The supernatant (post activation) obtained from PRP and PPP that could thaw at room temperature. The MILLIPLEX is based on the luminex Xmap technology, which performs immunoassays on the surface of fluorescent-coded beads known as microspheres [Millipore Corporation 290 Concord rd. Billerica]. IGF and P-Selectin ELISA kits were measured by ELISA (R&D Minneapolis Minn) according to manufacturer’s instructions. Briefly, standards of known concentration and PRP/PPP samples were added to a microwell plate with an antibody against each factor. Any growth factor present was bound by the immobilized receptor. Afterwards, any unbound substances were rinsed away, an enzyme-linked polyclonal antibody specific for each growth factor was added to the wells. After a second wash, a substrate solution was added, and the color developed in proportion to the amount of bound growth factor in the first step. The colour development stops once all the substrate have been bound, this was followed by measuring the colour intensity. On the other hand, concentration of TGF-beta1, EGF, VEGF, FGF-2, PDGF-AA and TGH-beta1 were measured with the MILLIPLEX ™ MAP kit. Samples were initially diluted 1:10 in assay buffer and retested at higher dilution if the median fluorescent intensity (MFI) was higher than the value of the top standard. Samples diluted 1:100 for these assays in the kit diluents. All samples were tested in duplicate. Manufacturer’s controls were running and found to fall in the range indicated for the kit.
Statistical Analysis
Statistical analysis was carried out using a linear model based on log transformed values of the growth factors. PRP and PPP groups for each of the diabetic and healthy groups separately. In addition, the mean difference, 95% confidence interval and the n-fold increase of PRP to PPP was derived. Relationships between platelet count and growth factors was investigated using a linear regression model. Correlations between growth factors and PRP count and GFs and platelet count were assessed by Pearson’s correlations. Normality checks and model checks were also carried out and no major violations of statistical assumptions were noted All analysis was carried out at the 5% (2 -tail) level of significance.
Results
Hematology Analysis of Platelet-Rich Plasma
Complete blood count analysis was performed on the whole blood and platelet-rich plasma samples from the six diabetic patients. The platelet separation system increased the platelet count on average from 334,400 ± 117,000 platelet/ µL to 1,995,000 ± 805,000 platelet/µL. The Platelet baseline value ranged from 130,000 to 500,000 platelet/µL. The platelet separating system resulted in an average 6-fold increase in platelet concentration. The platelet rich plasma group was significantly higher in platelet number than the baseline whole group, with a value p > 0.0005.
Quantification of Growth Factors
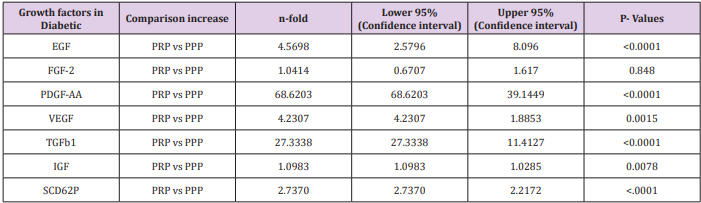
The quantification of the GFs from the PRP was determined in both the normal control and the diabetic patients. The expression of the GFs in both groups appeared similar. Conversely, when the expression of GFs from PRP were compared with the PPP; TGFbeta1, EGF, PDGF-AA and VEGF were significantly greater in diabetic patient and the healthy donors (Table 1a).
Comparison of Growth Factor Levels in Diabetic Patients (PRP vs PPP)
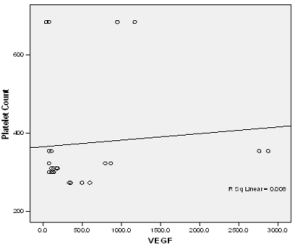
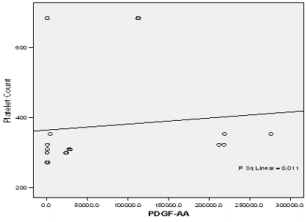
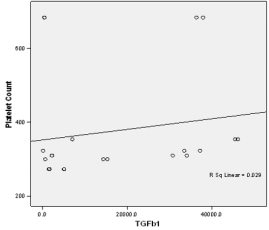
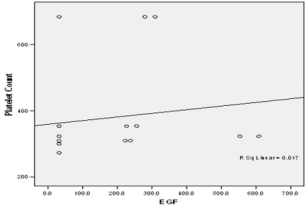
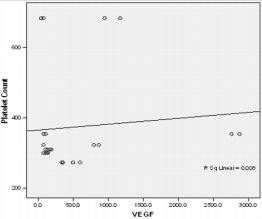
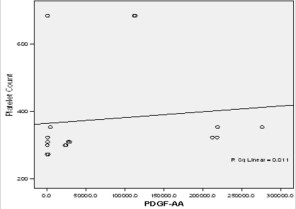
In this study PPP was used as the benchmark in which the n-folds increase for the GFs from the PRP were estimated. The mean content (±SD) of PRP versus PPP in EGF was 234.9± 193.8 pg/ml: 31.9 pg/ml with 5 fold increase, FGF-2; 49.2 ±33.64pg/ml : 49.2± 42.21pg/ml with no increase, PDGF-AA; 104.8± 101 ng/ml: 1.125± 0.674ng/ml had 68 fold increase; VEGF; 0.888 ± 0.97ng/ml: 0.167 ± 0.180ng/ml with a 4 fold increase, TGF-beta1; 28.384 ± 14.63ng/ml with a 27 fold increase. No significant was found in IGF in this study, IGF in PRP was 115.1± 32.54pg/ml: 106.1± 102.2 pg/ml in PPP. The level of platelet activation was estimated by the concentration of the P-selectin, there was two-fold increase in the value of the P-Selectin in PRP (161.5 ± 113.6pg/ml) compared to PPP(65.2 ± 17.67 pg/ml), which is statistically significant <0.0001 (Table 1b). GF expression in diabetics did not correlate with the platelet count nor with the PRP on the Pearson’s correlation coefficients analysis (rp) (r(p)- >0.2≤ 0.9). Relationships between platelet count and growth factors were investigated using a linear regression model, this was only performed for the four most significantly expressed GFs and the p value results are as follows: PDGF-AA;0.7157, VEGF;0.6616, TGF-1;0.3740, EGF;0.6499. None of the correlations were significant, and the correlations appear to be quite weak (maybe because of the sample size). A scatter plot of these GFs is shown in Figure 1a-1d with a linear regression line superimposed.Plots with Linear Regression Line superimposed for the 4 most expressed growth factors.
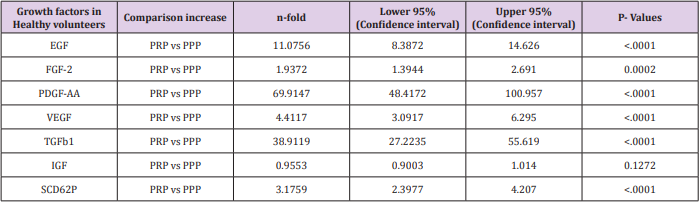
Table 1b: The expressions of growth factors were measured in duplicate for the 14 health volunteers.
Table 1a and 1b Comparison of expression of growth factors from PRP and PPP in both Diabetic patients and Healthy Donors with their level of significance.
Figure 1a: Linear regression of platelet concentrate against VEGf (vascular endothelial growth factor).
Figure 1b: Linear regression of platelet concentrate against PDGF-AA (Platelet derived growth factor –AA).
Figure 1c: Linear regression of platelet concentrate against TGF beta-1(Transforming growth factort beta-1).
Figure 1d: Linear regression of platelet concentrate against TGF beta-1(Transforming growth factort beta-1).
Comparison of Growth Factor Levels in Healthy Donor (PRP vs PPP)
A similar trend was noticed in the GFs expression in the healthy group, but the mean value appears higher in the healthy donor. The exception is the expression of VEGF and TGF-beta1 which are higher in the diabetic group. As explained above PPP was used as the benchmark in this study. EGF in PRP to PPP was (463 ± 259 pg/ml : 31.9pg/ml) which is a fold increase; FGF (136 ± 110.6pg/ml : 59.8 ± 48.98pg/ml), a 2 fold increase, PDGF-AA (123.6 ± 8.30ng/ml: 1.51 ± 1.04ng/ml) a 70 fold increase, VEGF (0.688 ± 0.808ng/ml: 0.136 ± 0.132ng/ml) a 40 fold increase and IGF (122.7 ± 20.89pg/ml: 128.4 ± 21.57pg/ml), no increase. The activities of P-Selectin estimating level of platelet activation of PRP (275.1±115.2pg/ml) are again two-fold higher compared with PPP (117.9 ± 129.8pg/ml). Table 1 showed the n-fold increase in the expression of the GFs in both diabetic patients and healthy volunteer as it relates to the chosen confidence interval of P=0.05. A linear regression analysis was used to determine whether a correlation existed between platelet count and growth factor concentration in the diabetic group. The correlation was done for the four most highly expressed GFs (VEGF, TGF, PDGF and EGF). Figure 2a-2d is the graphic illustration of the correlation. None of the parameters was statistically significant predictor of PRP count, this might be due to the small sample size or to apparently paradoxically low secretion of GFs in patients with very high platelet concentration. Surprisingly overall, there is no evidence of a linear relationship between platelet count and GFs expression.
Figure 2a: Plot of Platelet concentrate against VEGF.
The 4 graphs representing linear regression of the 4 most expressed growth factors with platelet count in diabetic patients.
Figure 2d: Plot of Platelet concentrate against EGF.
There is no evidence of a linear relationship between platelet count and the expression growth factor (the 95% confidence interval for the slope contains the value zero and therefore is not statistically significant) the p values are as follow PDGF -0.7157,VEGF-0.6616,TGF-1-0.374, EGF -0.6489.
Discussion
All measurements were performed in duplicate using validated commercially available ELISA and millipore kit, and coefficient of variance was <5%. For all analysis, the PRP/PPP initially generated was used stored in -80OC and was then activated just before analysis of the GFs. There are published studies that have shown that deep freezing is a safe method for releasing intracellular thrombocyte GFS [13]. The Platelets count from the diabetic patient’s serum and from the PRP were within acceptance range and corresponded to values previous reported in literature [14]. The correlation between the GFs and the platelet count from the PRP were weak, this might be due to small sample size, or might be due to isolated incidents of inefficient concentration of PRP. From the clinical point of view, it is important that the platelet concentration process is reliable by using a tested platelet separation device. The data obtained from this study further supports other published series, neither the direct platelet count nor the platelet in PRP could predict the desired expression of the GFs [15].
It is crucial to minimize platelet activation that occurs during PRP preparation, this is because activation results in untimely release of GFS, which could result in deletion of expressed GFs at the point of use. Furthermore, ability to store- frozen PRP has clinical implications from treating patients that might require repeated application of activated PRP in other to achieve the desire end point such as wound healing. In our study P-selectin was measured after activation of PRP as means of defining the degree of activation, and a 3-fold increase was noticed when PRP was compared with PPP in the healthy donor and the diabetic patient. In a similar study by Eppley et al. P-selectin was measured after centrifugation of PRP before the activation and they reported that there was no significant change in the level as compared with the whole blood [1]. Although our approach was different to the Eppley et al. the levels of GFs expression in our study compares favorably to other published series [4,15].
We compared the GF expression profile between PRP and PPP, contrary to the popular believe that PPP has no GF expression and only useful as a sealant [8], we noticed a significant expression of some growth factors from PPP, particularly from PDGF and TGF were the most prominent amongst the other expressed GF. Theoretically, levels of platelet-derived growth factors in PRP might be expected to depend on the number of platelets involved, which have previously been reported in some studies [15]. However, from our study we were not able to demonstrate a linear relationship. Eppley et al., concluded that the very best correlation was seen with TGF-beta1, which was still quite low, he suggested little correlation between platelet number and GF concentration and that content of the platelets varies from patient to patient. He further explained that this result might be due to by high individual variability in cellular production or storage of cytokines [5]. Of all the GFs identified in the wound cascade, the concentration of IGF-1 was not significantly increased. IGF-1 is primarily excreted by the liver into the blood plasma; hence the value of IGF is marginally higher in PRP than PPP in both groups. The findings in our study correlate with previous reports [1,6,15,16], which emphasized significant interpatient variability of growth factors expression.
The method of PRP preparation in our study achieved a significant level of GFs expression but there are practical drawbacks to this technique, limitation such as longer time of preparation and problem of fixed volume of whole blood needed to operate the device. However, it is debatable whether this is the most effective method, but it’s clinically convenient and user-friendly for the preparation process. In our study, there is an age and gender mismatch between our healthy volunteer and the diabetic group, however, other studies have also suggested that gender and age are not predictive factors in determining GFs expressions [16]. Further study with a control disease group will be more helpful to address the effect of chronic disease on GFs expression. Reliable prediction of GFs levels in PRP samples is necessary to ensure reliable and reproducible use of PRP for clinical treatment, since the regenerative potency of PRP undoubtedly depends on its GFs Level [17]. It is also therefore clinically relevant to recognize that each PRP processing method may differ as regards to platelet counts and activation techniques which may have implications for GF release and expressions [16].
Autologous derived Platelet rich plasma is promising novel technique that has found various applications beyond its initial application in periodontics and maxillofacial surgery [1,11,14]. It has been used in sport medicine with positive results in areas of cruciate ligament reconstruction, soft tissue injury, tendon and ligament injury. PRP have also been applied bariatric surgery, Brady et al. [18]. used PRP via an endoscopic delivery system, after laparoscopic Roux-en Y gastric bypass procedure to prevent bleeding and infection anastomotic leaks. He concluded that the use of PRP contributes significantly to wound healing and improved clinical outcome Furthermore, autologous fibrin derived from PPP, has been used as a biological sealant during aortic anastomosis during thoracoabdominal aortic surgery.
In a randomized control trial of endovascular repair of abdominal aortic aneurysm repair, with 50 patients in each arms of the treatment group, Saratzis et. al, reported a significantly lower postoperative hospital stay and lower inguinal wound-related complication [19]. Following clinical trials on the efficacy of PDGFBB (Becaplermin) in treating neuropathic diabetic foot ulcers, evidence suggests that bests results are obtained with PDGF-BB in combination with the aggressive debridement, a process which removes senescent fibroblast and pathogens from chronic wounds [4,20,21]. It was postulated that autologous derived Platelet-rich plasma has an advantage over the single recombinant GF, because the GFs have synergistic effect of the GF on each other and they promote mitogenesis of mesenchymal stem cells at the wound site [22]. The postulated therapeutic advantage has not been proven in any clinical trial.
Conclusion
Results from this study demonstrated that platelets can be sequestered and concentrated 6-fold from whole blood. Furthermore, PRP (either from diabetic patients or healthy volunteers) contains significantly higher levels of GFs expression compared with PPP, which has potential benefits on wound healing. Furthermore, we demonstrated that activating PPP will release similar expression of growth factors as seen in PRP but to a lesser degree. The prediction of GFs expression based on platelet counts have not been possible in this study. Further investigation might be needed to resolve the issues of unpredictability of growth factor expression from PRP. Furthermore, a technique whereby rapid quantification of GFs at the bedside may hold therapeutic potential.
For more Articles on : https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.