The Diagnostic Value of Galactomannan Testing in Bronchoalveolar Lavage Fluid on the Diagnosis of Pulmonary Aspergillosis in Patients with Chronic Respiratory Diseases
Introduction
According to the definition of chronic respiratory diseases
(CRD) by the World Health Organization, CRD is a group of diseases
that affect the airways and other structures of the lungs, the most
common include COPD, bronchial asthma, bronchiectasis, etc.
[1]. In-depth studies in recent years have found that pulmonary
aspergillosis can also occur in patients with chronic respiratory
diseases (CRD) [2,3]. As delayed treatment of pulmonary aspergillosis always leads to high mortality rate, early recognition
of CRD with pulmonary aspergillosis is extremely important.
Galactomannan (GM) is a thermally stable polysaccharide on
the cell wall of aspergillus filaments, which is released into the
blood from the tip of the mycelium during aspergillus growth [2].
GM can be detected in the blood in the early stages of infection.
Nevertheless, various factors have been found in clinical practice to
cause false positives and false negatives in galactomannan testing.
Bronchoalveolar lavage fluid (BALF) can be applied to detect
pathogens on lung lesions in the early stage of aspergillus infection.
Although BALF has been recommended for GM testing by domestic and foreign guidelines, there is no unified standard for BALF-GM testing cut-off value [2,3]. In this study, bronchoalveolar lavage fluid (BALF) was collected from 100 patients with suspected clinical pulmonary Aspergillus infections by means of bronchoscopy. BALF GM test and serum GM test were compared to assess the diagnostic value of galactomannan testing in bronchoalveolar lavage fluid on the diagnosis of pulmonary aspergillosis in patients with chronic respiratory diseases.
Patients and Methods
Patient Selection
Between June 2019 and December 2019,100 patients with suspected clinical pulmonary aspergillus infections from three different hospitals (50 patients from the Guangzhou Thoracic Hospital,45 patients from the Guangdong Province People’s Hospital, and 5 patients from the First Affiliated Hospital of Sun Yat-Sen University, respectively) were enrolled in this retrospective analysis. They all suffered from chronic respiratory diseases include COPD, bronchial asthma, bronchiectasis, etc. Data of all patients were collected, including age, sex, smoking history, past medical history and medication history, length of stay, laboratory tests, chest imaging examination, pathogen examination, lung pathology and bronchoscopy results. Serum and BALF GM tests were performed during their hospitalization. Factors that might cause false positives in the GM test such as piperacillin/tazobactam were excluded. Hematological malignancies, hematopoietic stem cell transplantation, solid organ transplantation, HIV infection, and patients with incomplete clinical data were excluded from the study.
Statistical Analysis
a. Koimogorov-smirnov test (K-S test) was used by SPSS 25
software to determine whether the target variables were
normally distributed. If the measurement data conformed
to normal distribution at the same time, it was represented
by mean ± standard deviation (`X±s). For the measurement
data that did not conform to normal distribution, it was
represented by M(P25-P75). The counting data was expressed
by percentage or constituent ratio. Independent sample T test
was used for BALF GM values of the case group and the control
group, paired sample T test was used for BALF GM values and
serum GM values of the case group, non-parametric rank-sum
test and Mann-Whitney U test were used for samples that did
not conform to normal distribution.
b. SPSS 25 software was used to draw ROC curves of the diagnostic
efficacy of BALF and serum GM test in the case group and the
control group, and the optimal cut-off value of BALF and serum
GM test for pulmonary aspergillosis was calculated.
c. The data of baseline features, clinical features and imaging
examination of the subjects were analyzed with the
independent sample T test or chi-square test for normal
distribution, and non-parametric rank-sum test for nonnormal
distribution. The differences were considered to be
statistically significant when p<0.05.
d. According to several guidelines, the cut-off value of GM was
between 0.5 and 1.5, and the cut-off value of 0.5, 0.8, 0.9, 1.0,
1.2 and 1.5 have been reported in many guidelines and metaanalyses.
Sen, spe, positive predictive value (PPV), and negative
predictive value (NPV) of BALF GM were calculated.
Results
Patient characteristics and data.4 patients with incomplete
data and follow-up loss were excluded, and a total of 96 patients
were included in this study. According to the diagnosis standards
of IDSA (2016) [2], 43 patients were diagnosed by pathological
data (proven diagnosis), and 3 cases were diagnosed by radiology,
etiology, and other clinical examinations (probable diagnosis). Both
of them were included in the case group. The control group included
6 cases of possible pulmonary aspergillosis and 44 cases of nonpulmonary
aspergillosis. Clinical data of patients were collected
(Table 1). The most common clinical symptoms in the case group
were cough (41cases,89.1%), hemoptysis (30 cases,65.2%) and
expectoration (27 cases,58.7%). Whereas in the control group were
cough (36 cases,72.0%), expectoration (27 cases,54.0%) and fever
(16 cases,32.0%). The clinical symptoms of hemoptysis and cough
were statistically different between the two groups.
The imaging findings of patients in the two groups included
nodular shadow, patchy shadow, consolidation shadow, air crescent
sign, cavity and aspergillus balls. Nodular shadow (27 cases,58.7%)
and cavity (22 cases,47.8%) were dominant in the case group,
while patchy shadow (14 cases,28.0%) and nodular shadow (13
cases,26.0%) were dominant in the control group. The imaging
manifestations of nodular shadow, cavity and aspergillus bulb were
statistically different between the two groups. Microbiological
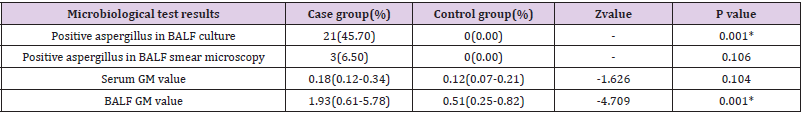
examination results. In the case group there were 21 cases (45.7%)
of positive aspergillus in BALF culture and 3 cases (6.50%) of
positive aspergillus in BALF smear microscopy. The serum GM
value was 0.18(0.12-0.34) in the case group and 0.12(0.07-0.21) in
the control group, showing no statistical difference. BALF GM value
was 1.93(0.61-5.78) in the case group and 0.51(0.25-0.82) in the
control group, Z value =-4.709. BALF GM value in the case group
was higher than that in the control group, P<0.05 (Table 2).
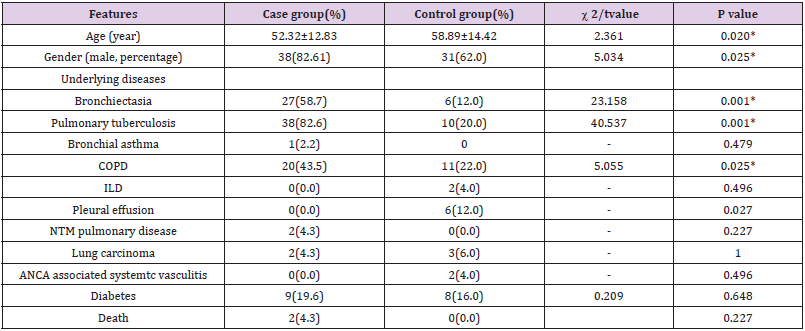
Table 1: Baseline characteristics of patients in case group and control group(%).
Note: *P value < 0.05, the difference was statistically significant
Table 2: Comparison of microbiological examination results between the case group and the control group.
Note: *P value < 0.05, the difference was statistically significant
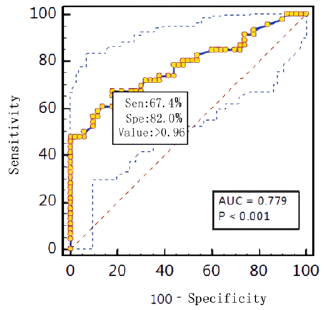
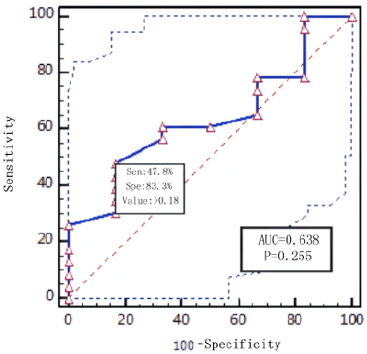
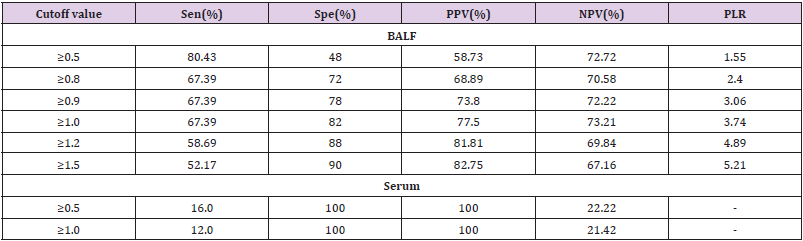
Diagnostic effificacy of the BALF GM test. When the GM cutoff value was 0.5,0.8,0.9,1.0,1.2,1.5, the sensitivity of BALF GM test decreased with the increase of GM cut-off value, and the specificity increased with the increase of GM cut-off value. When the diagnostic threshold of serum GM test was 0.5 and 1.0, the sensitivity decreased with the increase of the threshold, but the specificity did not change. BALF GM test had higher sensitivity but lower specificity than serum GM test (Table 3). The area under ROC curve of BALF-GM was 0.779(95%CI: 0.684-0.874),standard error was 0.0487,Z value was 5.727,P =0.001, Youden index was 0.4939,when thre hold>0.96,the sensitivity and specificity were 67.4%,82.0% respectively (Figure 1, Table 4). The area under ROC curve of serum-GM was 0.638(95%CI: 0.439-0.807), standard error was 0.121, Z value was 1.147, P=0.255, Youden index was 0.3116, when threshold> 0.18, Sen was 47.8%, Spe was 83.3%; When serum-GM threshold ≥0.18, AUROC was the highest, for which the sensitivity and specificity were 45.5%,83.3% respectively (Figure 2, Table 4).
Discussion
Structural lung disease is a major cause of pulmonary
aspergillosis, including bronchiectasis, PTB, bronchial asthma,
COPD, etc. Long-term and chronic diseases lead to the destruction
of the normal anatomical and physiological structure of the lungs,
the destruction of the mucosal barrier of respiratory epithelial
cells, and increase the ability of aspergillosis to adhere to airway
epithelium. In addition, cilium lodging and degeneration of airway
epithelium and obstruction of clearance of respiratory secretion
increase the chance of aspergillus infection [4,5]. This study also
confirmed that patients with pulmonary aspergillosis had more
chronic respiratory diseases in the case group than in the control
group (bronchiectasis 58.7% vs.12.0%, P=0.001),PTB (82.6
vs.20.0%,P=0.001),COPD (43.5% vs.22.0%,P=0.025),which was
consistent with the results reported in literature [6].The early
clinical manifestations of pulmonary aspergillosis are not specific,
and the typical chest CT findings are often related to the time of
disease occurrence and the severity of lesion development, and the
imaging findings cannot lead to a definite etiological diagnosis.
Traditional methods such as smear microscopy and fungal
culture have long cycle, low positive rate and are susceptible
to environmental pollution. Therefore,a variety of auxiliary
examination methods are used to achieve the purpose of early
diagnosis. Galactomannan (GM) is a specific polysaccharide of
aspergillus cell wall. At present, GM can be detected clinically by
blood, BALF, pleural effusion, cerebrospinal fluid and lung tissue, and
it is one of the common antigens for the diagnosis of aspergillosis.
A large number of existing studies have proved that the sen, spe,
ppv and diagnostic coincidence rates of BALF were higher than
those of serum GM. The results of this study showed that the cutoff
values of BALF GM test were all higher than serum GM, which
was consistent with the results of previous studies. The uniform
diagnostic threshold of BALF GM has been disputed at home and
abroad. The IDSA 2016 guidelines again recommended BALF GM
and serum GM tests as laboratory tests for pulmonary aspergillosis.
However, they did not specify a BALF GM value, but the diagnostic
threshold of serum GM test was ≥0.5[2]. In 2019, EORTC/MSGERC
scholars updated the definition of IFD, which clearly indicated for
the first time that the clinical diagnostic threshold of BALF GM as
pulmonary aspergillosis was: serum GM≥1.0,2 BALF GM≥1.0; or a
single serum GM≥0.7+a single BALF GM≥0.8 [7,8].
In this study, through ROC curve analysis, the AUROC of BALF
GM test was 0.779(95%CI:0.684-0.874).When BALF GM test limit>
0.96,Sen was 67.4%,Spe was 78.0%,PPV was 73.8%, NPV was 72.2%,
PLR was 3.06.When serum GM limit was greater than 0.18,AUROC
was the highest, Sen was 45.5%,Spe was 83.3%,and P=0.255.The
purpose of this study was to understand the value of BALF GM in
the early diagnosis of pulmonary aspergillosis in patients with nonneutropenia
complicated with pulmonary underlying diseases. Our
results showed that the sensitivity of serum GM test was lower
than BALF GM test regardless of setting GM≥0.5, ≥0.8, or ≥1.0 as
the diagnostic threshold of BALF GM. When GM threshold was≥0.5,
Sen,Spe,PPV of BALF GM were 80.43%,48.0%,58.73% respectively.
When the BALF-GM threshold was increased to ≥1.0, the PPV was
significantly increased. Compared with previous studies [9,10],
BALF GM values of patients with chronic respiratory diseases were
different from those of patients with traditional diseases such as
neutropenia, hematological malignancies, parenchymal organ
transplantation, hematopoietic stem cell transplantation, and
immunosuppressant use.
At present, some scholars have proposed that different
optimal diagnostic boundaries should be set for patients with
different underlying diseases and different immune states, such
as neutropenia and non-neutropenia [10], organ transplantation
(including hematopoietic stem cell transplantation) and non-solid
organ transplantation [11,12], hematological malignancies [13,14],
etc. Similarly, the interpretation of BALF GM test results should
also be based on the full assessment of the underlying diseases
and immune status of patients to determine the optimal BALF
GM diagnostic threshold for various patients, so as to improve
the diagnostic efficacy of BALF GM in the diagnosis of pulmonary
aspergillosis in different populations. Research and clinical practice
at home and abroad have found that many factors affecting GM tests
cause false positives and false negatives in GM tests, which often
confuses clinical work and even leads to misdiagnosis, missed
diagnosis and excessive antifungal treatment.
In this study, it was found that BALF GM value was higher in
some patients without aspergillus infection in the control group,
while BALF GM value was lower in a small number of patients
with aspergillus infection in the case group, resulting in false
negative in addition to sample dilution during BALF collection,
which might also be related to the use of antifungal drugs. A recent
review suggested that false negatives in GM tests were associated
with the use of antifungal active agents and myxolytic agents [15].
Using beta lactam classes of antibiotics (especially piperacillin/
he azole temple, amoxicillin/clavulanic acid potassium, etc.),
intravenous use of parenteral nutrition, blood product containers
containing glucose acid, severe gastrointestinal mucous membrane
inflammation, multiple myeloma will lead to GM false positive
[15]. Clinical cases have also reported that contamination of sterile
containers could lead to false positives of GM [16]. According to
previous studies and the results of this study, the early diagnosis of
pulmonary aspergillosis requires combining imaging examination,
histopathology, smear microscopy, fungal culture, aspergillosis
antigen detection, aspergillosis antibody detection, and molecular
biological examination.
Conclusion
In this study, BALF GM test is more valuable than serum GM test
for diagnosis. BALF GM test is more significant for the diagnosis of
pulmonary aspergillosis. The best limit, sensitivity and specificity
of BALF GM test are 0.96,67.4% and 82.0%(P=0.01). The optimal
threshold of BALF GM may vary with host-based diseases and even
with different species of Aspergillus. BALF GM value of pulmonary
aspergillosis under different immune states needs more clinical
data. At the same time, when serum GM and BALF GM are used in
clinical practice, it is necessary to fully understand and identify the
false positive and false negative of GM, and to diagnose pulmonary
aspergillosis by integrating patient factors, clinical manifestations,
imaging examination and pathogenic microbial examination.
For more Articles on : https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.