Dextromethorphan As A NOX2 Inhibitor Mitigates Endotoxemia-Induced Sickness Behavior and Brain Interleukin-1β Production in Mice
Introduction
Severe systemic infections (sepsis) can cause cytokine
storm and lead to acute brain inflammation through the overproduction
of proinflammatory factors, such as reactive oxygen
species (ROS), nitric oxide (NO) and cytokines release by activated
microglia [1]. Acute brain inflammatory responses might result
in both short-term behavior alterations (sickness behavior) and
chronic neuronal damage via sustained microglial activation [2-5].
Sickness behaviors associated with infections by bacteria, viruses or
fungi are common [6]. Sickness or sickness behavior refers to
a series of physiological and behavioral changes, which is thought
as a malfunction due to systemic inflammatory response during
infections or injury, including fever, fatigue, sleepiness, anorexia,
cognitive dysfunction, social withdrawal [7,8]. Although it might
be beneficial for individuals to fight the infection induced by
pathogenic microorganisms [8], immoderate sickness could further
aggravate its harm to the health, even death.
For example, neuronal excitotoxicity would be exacerbation
following persistence increased temperature in brain tissue during
fever, even destroy the blood–brain-barrier permeability [9,10].
Multiple cytokines such as IL-1β, IL-6 and TNFα are associated
with sickness behavior [7]. IL-1βseems to be the predominant
mediator of sickness behavior in the brain, because blockade of
its action by central administration of the IL-1 receptor antagonist
significantly attenuates sickness behavior [11]. The propagation
of neuroinflammatory cytokines during peripheral immune
challenge not only induces sickness behavior but also triggers
neuroinflammation characterized by the activation of microglia,
the resident macrophage in the brain [12]. Moreover, prolonged
neuroinflammation can cause long-term neuronal loss via the selfpropelling
vicious cycle between injured neurons and overacted
microglia [3,5]. Among various pro-inflammatory cytokines, IL-1β
showed a high potent neurotoxicity [13,14].
Our recent study has revealed that the IL-1β signaling is essential
for maintaining endotoxemia-associated microglial activation.
Reducing brain IL-1β levels prevents chronic neuroinflammation
and resultant neurodegeneration [15]. In this study, we
investigated the role of NADPH oxidase (NOX2) in LPS-elicited
sickness behaviors. NOX2, a major superoxide-producing enzyme,
plays a critical role in the development of neuroinflammation [5-
18]. We hypothesized that NOX2 is essential for sickness behavior
and brain IL-1β maturation during endotoxemia-mediated sepsis.
Besides NOX2-/- mice, we also employed a NOX2 inhibitor
dextromethorphan (DM), a widely used non-opioid cough
suppressant, which exerts anti-inflammation and neuro-protection
through the inhibition of microglial NOX2 [19,20]. In this study, we
found that genetic knockout of NOX2 or pharmacological inhibition
of NOX2 with dextromethorphan diminished sickness behavior in
the LPS peritoneal injection mice model.
Furthermore, the deficiency of NOX2 or the post-treatment of
dextromethorphan significantly decreased IL-1β production in the
brain and resultant microglial activation. This study demonstrated
that NOX2 plays a critical role in modulating the brain level of IL-
1β and associated sickness behavior. Our data also suggests that
dextromethorphan could be used in endotoxemia patients for
relieving sickness behaviors and neuroinflammation.
Materials and Methods
Animals and Treatment
The C57BL/6J mice and NOX2-/- mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Housing and breeding of animals were performed humanely with regard for alleviation of suffering following the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011). LPS (2.5mg/kg) or saline (2.5 mg/kg) were intraperitoneally administrated to 8-week male C57BL/6J mice and NOX2-/- mice initially, and sickness behavior was observed every 3hs after LPS injection until 24h. Dextromethorphan (12.5mg/ kg) or saline (12.5mg/kg) were also intraperitoneally injected to C57BL/6J mice two times (3h and 9h after LPS injection) and sickness behavior was accessed every 3hs after LPS injection until 24h.
Sickness Behavior Assessment
We applied behavior test to evaluate the mice’s sickness
behavior after treatment, including the response, the moving
distance, ptosis, piloerection and excretion in eyes.
a) Response. The toughing frequency until the mice moved. We
have four degree: 0. Animal moved freely without stimulation.
1. The movement of mice under one- or two-times’ toughing
stimulation. 2. Three to five toughing stimulation. 3. More than
five stimulations.
b) Moving distance. We also set four degree to assess moving
distance. 0. The animal moved freely more than 20cm without
any stimulation. 1. The moving distance is more than 20cm
with stimulation. 2. The moving distance is 10cm to 20cm
under toughing stimulation. 3. In case of mice moving a few
steps with stimulation, they were regarded as grade 3.
c) Appearance of ptosis, piloerection and excretion in eyes. 0.
Negative. 1. Positive.
Immunohistochemistry
Mice were euthanized using fetal plus at the desired time points after injection. Brains were fixed in 4% paraformaldehyde and processed for immune staining as described previously [21,22]. We used the following primary antibodies for immunohistochemistry: antibodies against CD11b (1:400, AbDSerotec, Raleigh, NC). Immuno-staining was visualized by using 3,3’-diaminobenzidine (DAB) and urea-hydrogen peroxide tablets.
Quantification of the immunohistochemistry staining density
The quantification CD11b immunohistological staining in SN was performed by Image J software based on a protocol for quantifying western blots [23]. Briefly, the image was first converted into the grayscale picture, and the background was adjusted before the quantifying area was selected for the measurement of the total pixels. The relative density of the staining was compared based on the density of the total pixels of a certain brain region (total pixels/ area).
Cytokine Analysis
The brain tissues from WT and NOX2-/- mice were measured for mouse IL-1β (R & D Systems Inc.,Minneapolis,MN) according to the manufacturer’s instructions.
Real-time RT-PCR Analysis
Total RNA was extracted from the mouse brain by using a RNeasy Mini Kit from QIAGEN (Valencia, CA) to detect the level NOX2 (gp91) according to the previous description [4]. Total RNA was reversely transcribed with MuLV reverse transcriptase and oligo-dT primers. Then SYBR green PCR master mix was used for realtime PCR analysis. The primer sequences were as follow: GAPDH F (5′ TTC AAC GGC ACA GTC AAG GC 3′), GAPDH R (5′ GAC TCC ACG ACA TAC TCA GCACC 3′), NOX2 F (5′ GAT TCAAGA TGG AGG TGG GAC 3′), NOX2 R (5′ GGT CAG TGT GAA TGG GTG CC 3′). Real-time PCR amplification was performed using SYBR Green PCR Master Mix and QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) according to manufacturer’s protocols. Amplifications were done at 95°C for 10 s, 55°C for 30 s, and 72°C for 30 s for 40 cycles. All samples were tested in duplicate and normalized with GAPDH using the 2-ΔΔCt method. Fold changes for each treatment were normalized to the control.
Statistical Analysis
All data were represented as mean ± SEM. Data were analysed using one-way or two-way ANOVA. Tukey’s post hoc tests were applied when comparing groups to only the control, or Sidak’s post hoc test when comparing between multiple treatment groups. Number of mice used is indicated in each figure legend.
Results
NOX2 Plays a Critical Role in Mediating LPS-Elicited Sickness Behavior
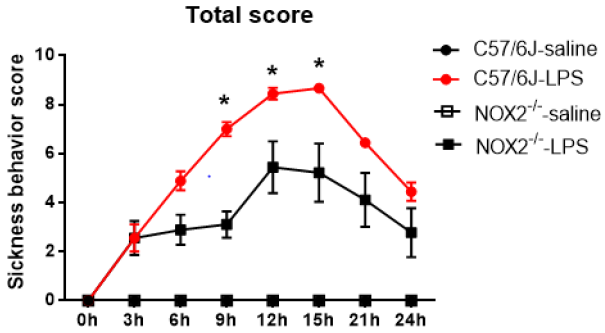
C57BL/6J and NOX2-/-mice received a single injection of LPS (2.5mg/kg; ip) for investigating the role of NOX2 in the development of LPS-induced sickness behavior. Sickness was assessed every 3hs after LPS administration up to 24h. In WT mice, the sickness behavior score showed a time-dependent increase over time, peaked around 9-15h following LPS injection, then declined gradually afterwards, but still remained higher than the base line values at 24h after LPS injection. A similar pattern of change in the sickness behavior score was observed in LPS-injected NOX2-/- mice. However, a significant reduction in sickness behavior score in NOX2-/- mice was observed at 9h,12h,15h, compared to WT mice (p<0.05; Figure 1).
Figure 1: The total scores of sickness behavior in different groups after LPS administration. NOX2-/- mice (N=9) displayed attenuated sickness behavior after LPS challenge compared to WT mice (N=9). Sickness behavior scores were measured every 3hs until 24h after LPS i.p. injection (2.5mg/kg) in WT and NOX2-/- mice.*p<0.05, the mean differences in total score between two groups was observed at 9h (p<0.0001), 12h (p=0.0405) and 15h (p=0.0382) after LPS treatment. Repeated-measure ANOVA and multi¬variate ANOVA to test the differences over time and differences between groups.
The absence of NOX2 Suppresses Brain Levels of IL-1β
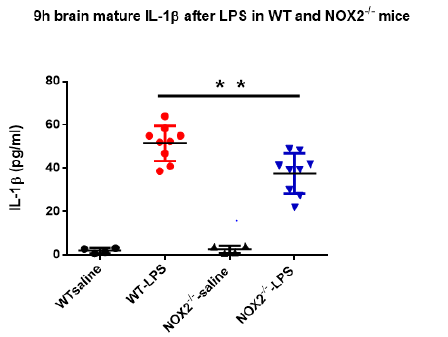
As mentioned previously, increase in brain IL-1β plays a critical role in mediating sickness behavior. Our previous study indicated that the production of mature brain IL-1β in brain peaked around 9h after LPS injection [15]. Since the sickness behavior analysis showed high scores at 9h upon the peritoneal LPS stimulation (Figure 1), we measured mature IL-1β concentrations in brain tissues at 9h in both WT and NOX2-deficient mice. Results showed that level of IL-1β in brain tissues of WT mice was higher after LPS injection than that in vehicle-treated mice. Notably, the level of IL- 1β in brain tissues from NOX2-/- mice after LPS administration was significantly lower than that of LPS-treated WT mice (Figure 2). This result indicates that NOX2 is essential in mediating LPSinduced increase in the IL-1β production.
Figure 2: The brain mature IL-1β at 9h after LPS in WT and NOX2-/- mice. Mature IL-1β concentrations in brain tissue were measured by ELISA at 9h after LPS treatment and they were decreased in NOX2-/- mice compared to WT mice after LPS injection (2.5mg/kg) (n=4-9/group). **p<0.05, the differences of IL-1β concentrations between LPS-treated WT and NOX2 groups. Two-way ANOVA followed by Sidak’s multiple comparison test. (treatment×group, F(1,22)=5.152, p=0.0334; effect of groups, F(1,22)=4.434, p=0.0469; effect of treatment, F(1,22)=177.3, p<0.0001).
The Deficiency of NOX2 relieves LPS-Elicited Activation of Microglia in The Substantia Nigra
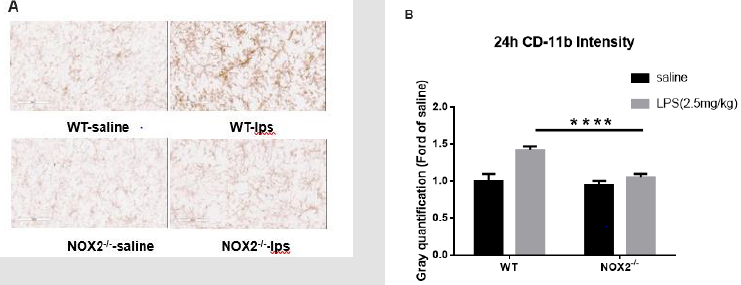
The reduction of brain mature IL-1β prevents sustained microglial activation induced by endotoxemia [15]. Therefore, we examined microglial activation in the absence of NOX2. Because sepsis-associated neuroinflammatory responses involve multiple brain regions and the substantia nigra (SN) was found a good area in our previous study to observe the persistence of microglial activation [3,4], we chose the SN in this study to evaluate the degree of microglial activation by immune-staining analysis. Typical morphological changes reflecting microglial activation were observed in WT mice 24h after LPS injection. Activated microglia exhibited significant enlargement of cell bodies, irregular shapes. This morphological activation was significantly reduced in NOX2 deficient mice, characterized by a small and round shape compared with WT mice. Analysis by gray quantification showed pattern changes similar to those of morphological observations (Figure 3a & 3b).
Figure 3: Microglia activation in SN was detected with CD11b IHC at 24h after LPS (2.5mg/kg) treatment.
(a) Attenuated microglial activation in NOX2-/- mice compared to WT mice. Following a single injection of LPS (2.5 mg/kg;
ip) or saline vehicle, mice were perfused 9 h thereafter for microglial CD-11b immunostaining (n = 3/group). Representative
images at 9h were shown (Figure 3A). Scale bar = 300 μm.
(b) CD-11b density was quantified. Results are expressed as folds of
vehicle control. Histograms represent degree of microglial
activation quantified by measuring the density of CD-11b staining with
ImageJ. ****p<0.0001, the differences of gray
quantification between LPS-treated WT and NOX2-/- groups. Two-way ANOVA
followed by Sidak’s multiple comparison
test. (treatment×group, F(1,68)=20.45, p<0.0001; effect of groups,
F(1,68)=35.93, p<0.0001; effect of treatment, F(1,68)=56.21,
p<0.0001).
Targeting NOX2 as a potential therapy for sepsis-related sickness behaviors
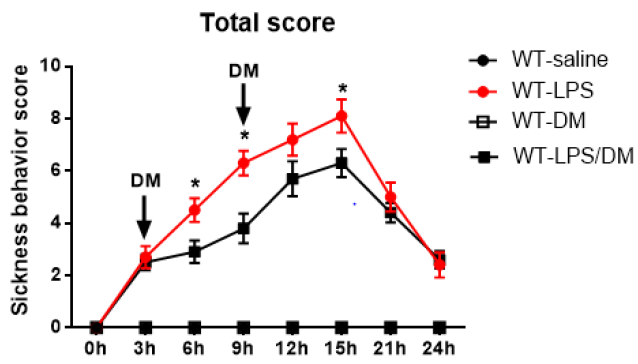
Results from NOX2-deficient mice suggest a potential target for developing drug therapy for sepsis-related sickness behaviors. As proof of principle, we employed dextromethorphan (DM), which displays potent anti-inflammatory property via inhibition of microglial NOX2 activity [20,24]. At 3h and 9h after mice received a LPS injection, DM was intraperitoneally injected and sickness behavior was accessed every 3hs for 24hrs. In LPS treated mice, the sickness behavior score increased gradually up to15h after LPS injection, then it decreased significantly until the end of observation. Analysis of ANOVA showed that DM significantly attenuated sickness behavior at 6h,9h, and 12h compared to mice with LPS treatment alone (p<0.05; Figure 4).
Figure 4: Post-treatment of dextromethorphan (12.5mg/kg) mitigatedLPS-induced sickness behavior in WT mice (N=5-10 for each group). Sickness behavior scores were measured every 3hs until 24h after LPS i.p. injection (2.5mg/kg) and indicated administration of dextromethorphan. *p<0.05, the mean differences in total score between two groups was observed at 6h (p=0.0459), 9h (p=0.0411) and 15h (p=0.0483) after initial LPS treatment. Repeated-measure ANOVA and multi¬variate ANOVA to test the differences over time and differences between groups.
DM reduces LPS-elicited the IL-1β production, NOX2 mRNA level and diminishes the microglial activation
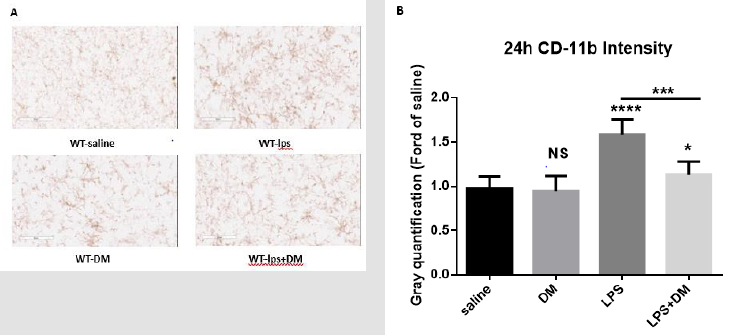
LPS-elicited increase in IL-1β production from brain tissues and microglial activation were determined in WT mice. Similar to the results from NOX2-deficient mice, DM treatments effectively reduced LPS-induced IL-1β production (Figure 5a). NOX2 mRNA levels increased significantly following initial LPS treatment. While the NOX2 mRNA expression in DM post-treatment groups at different timepoints (6h and 12h)were lower than LPS-treated alone (Figure 5b). Pathologically, DM diminished the activation of nigral microglia as well (Figure 6a & 6b). Taken together, these results suggest a potential use of DM in ameliorating the severity of sickness behaviors and neuroinflammation in endotoxemia-related patients.
Figure 5: Post-treatment of dextromethorphan alleviated brain mature IL-1β levels and NOX2 mRNA expression.
a) Post-treatment of dextromethorphan alleviated brain mature IL-1β levels. (LPS 2.5mg/kg) (n=5-10/group). *p<0.05, the
differences of IL-1β concentrations between LPS-treated and post-treated dextromethorphan groups. One-way ANOVA
followed by Tukey’s multiple comparison test. (F=32.6, p<0.0001).
b) (NOX2 mRNA levels were measured by qPCR at 6h, and 12h after LPS treatment. DM was post-added to mice at 3h and
9h after LPS treatment, respectively. #P<0.05, ##P<0.01 for the differences of NOX2 mRNA level between LPS-treated and
post-treated DM groups at 6h and 12h, NS for 9h. One-way ANOVA followed by Tukey’s multiple test (F(3,32)6h=31.42,
F(3,32)9h=29.57, F(3,32)12h=32.66). **P<0.001, the differences of NOX2 mRNA level over time in both LPS -treated groups and
post-treated DM groups. Repeated-measure ANOVA and multivariate ANOVA test the difference over time and differences
between groups.
Figure 6: Post-treatment of dextromethorphan alleviated brain microglia activation in SN.
a) The microglia activation in SN was detected with CD11b IHC at 24h after LPS treatment. (n = 3/group) (LPS 2.5mg/kg, DM
12.5mg/kg). Representative images at 9h were shown (Figure 6A). Scale bar = 300 μm.
b) CD-11b density was quantified. Results are expressed as folds of
vehicle control. Histograms represent degree of microglial
activation quantified by measuring the density of CD-11b staining with
ImageJ. One-way ANOVA followed by Tukey’s
multiple comparison test. (F=62.73, p<0.0001). ***p<0.001, the
differences of gray quantification between LPS-treated and posttreated
dextromethorphan groups. ****p<0.0001, *p<0.05 and NS compared to
vehicle group.
Discussion
This study provides the first evidence indicating that microglial
NOX2 plays a critical role in endotoxemia-related sickness
behaviors. Mechanistically, inhibition of microglial activation and
subsequent reduction in IL-1β production through the inhibition of
NOX2 underlie the amelioration of LPS-elicited sickness behaviors.
This conclusion was based on both genetic knockout of NOX2 gene
and pharmacological inhibition by DM. This study also suggests
that microglial NOX2 can be a therapeutic target for developing anti
sickness behaviors drug. In present study, we found that mice with
NOX2 subunit knockout exhibited lower sickness behavior score
than WT mice after LPS administration. Furthermore, compared to
WT mice following LPS treatment, reduced activation of microglia
(24h after LPS treatment) and decreased production of proinflammatory
IL-1β (9h after LPS treatment) were both observed
in this study. Mechanistically, enhanced production of NOX2-
generated superoxide and its related ROS is regarded as the major
source of pathological oxidative stress in CNS leading to neuron
damage, inflammation, and dysfunction.
In the current study, the NOX2 deficiency reduced acute
production of IL-1β and activation of microglia, indicating that the
NOX2 deficiency could attenuate the LPS-induced acute symptom
via reducing acute neuroinflammation. After elucidating the role
of microglial NOX2 in mediating LPS-induced sickness behaviors,
as a proof of principle we further provide evidence showing high
efficacy of DM in ameliorating sickness behaviors. In addition,
post-treatment with DM following LPS also attenuated microglia
activation, IL-1β production, as well as NOX2 mRNA in vivo. Over
decades, numerous studies demonstrate that DM, a non-opioid
cough suppressant, has neuroprotective effects, which is attributed
to its antagonistic effect on NMDA receptor [25-27]. However,
we previously reported that anti-inflammatory effect of DM is
attributed by inhibiting preventing microglia over-activation [28]
induced by LPS and MPTP–elicited neurotoxin in neuron-glia
culture and in vivo studies [29].
Furthermore, these mechanistic studies revealed that DM
reduced the NOX2-generated superoxide free radicals from
microglia, which in turn reduced in production of iROS and
cytokines. The ill patients always have representative characters
in behavior and/or emotion, including fever, fatigue, anorexia,
fragmented sleep, losing interesting in physical and social
environment, even showing emotion disorders such as depression.
Over past two decades, a term of “sickness behavior” is suggested
to describe such changes mentioned above in ill individuals or
animals. Many studies have clarified the underlying mechanism
that pro-inflammatory cytokines, such as IL-1α, IL-1β, TNF-α, IL 6, play
a critical role in sickness behavior and CNS dysfunction
[30,31]. Despite moderate inflammation or sickness behavior is
beneficial for coping with foreign microorganism-elicited infection,
the uncontrolled cytokine storm or serious sickness behavior
could impair the immunity system, interfere with inflammatory
responses and produce further damage to patients.
Therefore, from clinical perspective, it is desirable for ill
patients to control or relieve excessive inflammation and sickness
behavior after proper anti infection therapy. Our results suggested
that DM and NOX2 deficiency not only inhibited activation of
microglia, reduced sickness behavior, but also suppressed NOX2
gene expression and production of IL-1β, which is also a critical
pro-inflammatory cytokine in neuroinflammation. IL-1β plays a
significant role in the initiation of inflammatory response in CNS
and transition to chronic neuroinflammation induced by various
stimulation [32]. IL-1β could impair the integrity of BBB, leading to
infiltration of peripheral immune cells into brain, and aggravating
the neuroinflammation [33]. Meanwhile, IL-1β activated microglia
and astrocytes, which in turn generate other pro-inflammation
cytokines, such as IL-18, TNF-α, IL-6 in CNS. Furthermore, the IL-
1β indirectly recruits leukocytes by augmenting the expression of
chemokines.
These mechanistic studies indicated the IL-1β is a critical
inflammatory cytokine in both acute and chronic inflammatory
response in CNS. Thus, this study not only demonstrates a
beneficial effect of inhibiting NOX2 in mitigating the sickness
behavior symptoms during acute inflammation, but also can
be beneficial in preventing the transition from acute to chronic
neuroinflammation. Over the years, a number of studies have
reported that DM has neuroprotective effects [25,34-36]. The
mechanism of neuroprotective activity has been attributed to
DM’s potential antagonistic effect on the NMDA receptor complex.
Recently, studies indicated that DM also play neuroprotective roles
in cognition deficit following systemic inflammation [37], together
with previous reports [19], suggest that DM achieves these
extraordinary protective effects through the inhibition of NADPH
oxidase. Our study demonstrated DM and NOX2 deficiency attenuate
the microglial activation during acute inflammation, meanwhile,
the reduced IL-1β production and NOX2 gene expression in brain
also was observed. Subsequently, the sickness behavior induced by
LPS relieved following acute inflammation.
This finding is similar with the protective effect of DM in chronic
neuroinflammation [18,20]. Several studies have investigated the
role of a variety of pro-inflammatory cytokines such as TNF-α,
IL-1, IL-6, IL-8, IL-10 and IFN-γ and reactive oxygen species like
superoxide and iROS in the pathogenesis of sepsis or infection.
To relieve inflammation in septic or infected patients via blocking
single cytokine (e.g., IL-1β receptor antagonist), while the crosstalk
in cytokine network makes this effort uncertain and controversy
[38-40]. The present study offers a novel approach by using DM
to attenuate infection-related sickness behavior, which selectively
targets NOX2. Furthermore, the findings from this study provide
a potential therapeutic viewpoint: DM would be an alternative
drug for septic or endotoxemia-related sickness behavior besides
antibiotics and other life supportive therapy, such as ventilation,
oxygen, fluid replacement therapy. In conclusion, DM attenuated
sickness behavior in mice induced by LPS via inhibiting NOX2
activation and suppressing the production of IL-1β in brain. The
present findings demonstrated that NOX2 is a novel therapeutic
target for sickness behavior. Furthermore, this study also suggests
that owing to its excellent safety record and high efficacy, DM has a
great potential to be tested in clinical settings.
For more
Articles on : https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.