Experience of Indian National Biobank in COVID-19 Pandemic and Future Directions
Introduction
The Coronavirus disease 2019 (COVID-19) is an infectious
disease caused by the Severe Acute Respiratory Syndrome
Coronavirus-2 (SARS-CoV-2) [1]. SARS-CoV-2 was first reported
in China and till date accounted for 169 597 415 confirmed global
cases of COVID-19, including 3 530 582 deaths. Worldwide [2].
On March 11th 2020, WHO declared public health emergency
[3]. High transmissibility of this virus rapidly surged number of
cases and many countries around the globe announced regionalnational
lockdowns [4]. The lockdown situations adversely affected
businesses, slow down of scientific activities and operational
activities of several sectors including biobanks [5]. The National
Liver Disease Biobank (NLDB), India is an advanced open resource
sharing liver disease biobank for liver and associated disease
research established with the joint efforts of the Department of
Biotechnology (DBT) and Institute of Liver & Biliary Sciences (ILBS),
New Delhi, Government of India [6]. NDLB is India’s first liver
disease biobank with a storage capacity of more than 5.4 million
biosamples and certified by Tissue Repository Network (CTR.
Net) in 2020 [7]. NLDB has been set up in an institute dedicated to
patient care and research in liver diseases.
The biobank collects high quality biosamples across the country
with clinical data. A total of 73,831 aliquots of serum, plasma, PBMC,
urine, tissue, stool, and whole blood from 12,607 patients have
been collected and stored at NLDB as of Dec 31st 2020. Biosample
and access to the advance analytical facility openly available
under one roof for all researchers. In order to deliver cutting edge
services for collaborative liver disease research NLDB acquired a
non-profitable business and financial model, charging only the
cost for utilization of services, NLDB engaged trained and highly
competent staff with world class storage and advanced analytical
infrastructure, aiming to become a nodal centre for providing the
clinical and basic researchers to reliably store biosamples and carry
out their research at one platform. The national sudden lockdown
was placed on 24 March 2020 in India for 68 days in different
phases when the number of confirmed coronavirus cases were
approximately 500 [8]. The lockdown restricted people to stay in
their homes [9] and all transport services were suspended with
exceptions for essential emergency services [10].
Impacts
The sudden lockdown brought both the opportunities and challenges to the biobank. Although, the National Liver Disease Biobank (NLDB) is a liver and related diseases biobank, the government of India designated it as an add-on COVID biobank permitting for collection and storage of COVID-19 biosamples for research, developing diagnostics and vaccines. NLDB faced tri-directional challenges based on financial, operational and sustainability, but were accepted positively with changing in the processes and management.
Crisis Management
The storage facility and associated equipment are one of the key elements in operations of biobank. As per best practices published by International Society for Biological and Environmental Repositories [11], telephone numbers for professional assistance should be clearly posted in the repository and accompanying administrative areas (e.g., engineering or facilities personnel, power companies, fuel supply companies, transportation services). The emergency planning was focused to maintain cryopreservation of biosamples from various possible events that may breakdown the freezers. NLDB has 10 % of the total storage capacity as backup, maintained at operating temperature at all times. Safe guarded by 24x7 CCTV surveillance and a security personal and all mechanical freezers connected with datalogger equipped with SMS alert system. Three biobank personnel are trained and even prepared for 24x7 shifts in case of emergency. Contact numbers of emergency response team (engineering, electricity and security office) are posted on all storage units. Earlier the emergency plan was only focused for natural calamities. Learning from the current situation, an upgraded emergency plan based on management and transportation of sample at satellite center, business strategy, financial planning and operations of biobank is under review. Moreover, NLDB also started to develop contingency plan to keep operating in pandemic positions. There were difficulties in taking consent with COVID infected patients. Leftover diagnostic samples stored at biobank without consent will be utilised for research after approval from ethics board.
Sample Collection
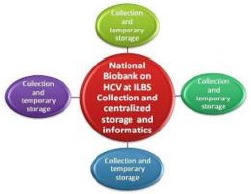
The NLDB follows the “decentralized collection, centralized storage, distribution and informatics” model. (Figure 1). It has collaboration with 18 hospitals for collection of biosamples and supports many research projects by providing biosamples along with associated data. Biosamples are collected with necessary precautions, however, in this pandemic, the need of PPE kit, sanitizer, and establishment of BSL2/BSL3 facility was critical, considering all samples as highly infectious.
The challenges confronted while functioning in this pandemic:
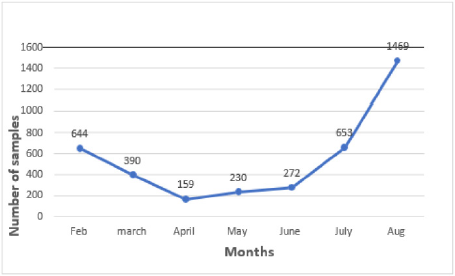
1. There has been a significant decline in the number of samples
collected from both host institute and the satellite centres
because Outpatient Department and surgeries are only limited
for emergency cases (Figure 2). 2. Co-ordination with satellite
centers and maintenance of samples became difficult because
of limited staff.
2. At initial period, hospitals were not prepared to screen for
COVID-19 for all patients, leading to high chances of collecting
COVID-19 contaminated samples from asymptomatic patients.
Sample processing protocols were revised and precautions
were made even for handling samples apparently COVID
negative.
3. Biobank was instructed to collect COVID-19 biosamples but
processing and storage area was not designed to handle highly
infectious samples. To avoid cross contamination, urgent
requirements for separate space for processing and storage of
COVID and non-COVID samples was flagged.
4. Dedicated routes to transport cryoshippers containing
aliquoted COVID biosamples were made from patient ward to
BSL2+ facility and then to the storage area.
Logistics and Supply of LN2 Gas
The surging Covid-19 in March, 2020 and the Indian government’s decision to contain the disease outbreak through lockdown adversely affected the domestic logistics sector, especially road transportation, production and supply of essential goods [12]. With increasing number of active cases of Covid-19, the consumption and demand of oxygen was increased throughout the country [13]. Some LN2 industries directed to produce more oxygen in comparison to LN2 gas. The resource management for consumables, refilling of LN2 in cryoshippers, transient storage and transportation of biosamples are managed from main centre established at New Delhi, India. The sudden nationwide lockdown almost got NLDB in a standstill affecting the operational chain such as managing the collection, storage, transportation of biosamples from satellite centres. Biobank has consumption of 100 litres/day to maintain temperature of two LN2 tank. NLDB does not have LN2 plant and dependents only on LN2 supply from outside. Closedown of LN2 factories due to movement of labours, local shortage/limited access to liquid nitrogen, shortage of drivers, made it difficult to get the LN2 tanks refilled. Moreover, the market price of LN2 was hiked up to three times in comparison to the previous routine rate. The pandemic taught that biobank should have inhouse plant for LN2 supply. To avoid such problem in future, NLDB processed to establish an LN2 plant in ILBS premises with capacity to produce approx. 250 litres of LN2/ day (Figure 3).
Operations
The ban and restrictions on public transport effected the employees resulting in only 40% attendance of the staff. Biobank staff were seconded in COVID-19 testing lab and the sudden focus and orders to quickly set up procedures to test covid-19 samples, and two-technicians infected with COVID-19 at different time periods and others quarantined for coming in close contact, were big challenges faced. IT experts were not able to resolve the technical issues in the biobank software from home due to nonavailability of remote access for the software. There was temporary interruption of collection and distribution activities as hospitals redirected to treat critical cases and COVID -19 patients only. During lockdown, one of the -80⁰C freezers stopped working, consequently the samples were shifted to the backup freezer. Repair was delayed due to restricted movement and limited supply of spare parts and backup LN2 freezer was being utilised for storage of COVID-19 samples. Biobank samples were on complete risk in case of any failure in storage system as the backup freezers were already in use. Emergency purchase of two -80 freezers was done to accommodate more COVID samples as left over covid-19 samples from hospital diagnostic centers were directed to store in biobank for future research. SOPs were revised as per the knowledge gained in the pandemic. A separate SOP is developed as per guidelines of Indian Council for Medical Research/ Government of India for collection, storage and distribution of COVID-19 samples for research.
Personnel Wellbeing
Commuting for the personnel was big issue in lockdown. However, staff working in COVID lab were provided accommodation in hospital. The safety guidelines issued by Ministry of Health and Family Welfare Government of India to maintain social distancing at work place and transport were followed with necessary compliance [14,15]. Routine test, thermal scanning, sanitizing machine, touch free mechanism installed at all entry and exit points and common areas. Complete ban on non-necessary visit and emergency visits were allowed only after negative rapid antigen test. Two biobank technical staff resigned from their job because their family not allowed them to work on COVID-19 samples.
Management Related Issues
a. Finance: A project for add-on COIVD biobank facility was
submitted to the Government of India which was approved and
funds released on priority basis in December, 2020.
b. Biobank Information Management System (BIMS): IT
related issues were impediment for biobank due to no remote
access of clinical databases, biobank systems, slow adaptation
and update of software. BIMS was updated with annotation for
COVID-19 as per recommendations of ICMR, GOI.
c. HAZARD Management: The primary and basic requirement
of biobank is safety of its staff and of the environment against
biological and chemical hazards. There were no specific
guidelines available for storage, collection, distribution and
QMS of highly infectious samples in ISO20387, NCI and ISBER
best practices. Sharing of COVID biosamples are not as easy
as non-COVID samples thus National Oversight Committee
was constituted by ICMR to review the same. NLDB has
provision to share the sample after approval of Biosample
release committee (BRC). Sample are released after signing
MTA and undertaking by recipient to handle COVID-19 and it
is informed that any violation or misuse would be dealt with
strict action as per laws of Government of India.
Work Culture & Infection
Work culture of biobank has been totally changed due to COVID fright and implementation of new rule and SOPs. Handling the informed consent, annotation forms duly signed by COVID patients was a big issue. WHO and ICMR guidelines are being followed by NLDB to prevention from any infection, Intensive communication and training on good hygiene practices, PPE kit donning and doffing has been provided to biobank personnel. Technicians are equally divided for COVID and non-COVID related work. It is compulsory to wear N95-type masks, use of hand sanitizer, disinfect all documents coming through patents in Ultraviolet (UV) light, and to sanitize work area daily and disinfect the storage area twice a week.
Research Support
The government of India has released huge funds for research focused on Diagnostics, Vaccines, Novel Therapeutics, Repurposing of Drugs or any other intervention for control of COVID-19 as most of the research institutes were closed or had limited access to maintain necessary equipment during lockdown.
Discussion
The sudden lockdown consequent to the COVID-19 pandemic brought both the opportunities and challenges to the biobank. NLDB handled the tri-directional challenges that were operational, financial and sustainability. Sudden changes in operations, supply chain disruptions, manpower presence and remote access of software were major difficulties along with the Handling of Covid-19 biosamples, inaccessibility of donors and challenges in obtaining informed consent. Although, there was neither biobank practices and standards included any plan to run a biobank in a pandemic, NLDB followed the available national [15] and international standards [16] and guidelines [11,17,18] to handle the infectious samples. Though, biobank had an emergency plan for backup storage though there were no thoughts to have an emergency plan for LN2 supply and to work with limited man power. Flexibility in purchase rules, monitoring of efficient utilization, stock management for every one month can be a great help to run biobank in emergency. Biobank must have inhouse LN2 plant along with a rate contract with suppliers to supply LN2 in emergency at equivalent prices. All SOPs revised to treat all sample as infectious Remote monitoring and access of software during emergencies is a must. However, development of remote monitoring software is only possible after the contribution of key stakeholders, such as hospital administration, IT team, privacy legal expert and biobank operations team. In conclusion, NLDB used this pandemic as a learning experience and modifying its operational, emergency and business plans for future crisis and pandemics.
For more
Articles on : https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.