Impact of Air Pollution on Semen Quality: The Specific Situation of Terni (Central Italy)
Introduction
Infertility is a prevalent condition affecting an estimated 72, 4
million people globally that is well recognized by The World Health
Organization (WHO). Although prevalence data are lacking, 9% of
couples struggle with fertility issues and male factor contributes
to 50% of the issues. Many genetic and lifestyle factors have been
implicated in male infertility; however, about 30% of cases are still
thought to be idiopathic [1]. The mechanism by which medical
conditions affect fertility includes effects on hormonal levels,
impairment of sexual function (including ejaculatory function),
or impairment of testicular function/spermatogenesis. In the
last 70 years, a decrease in sperm fertility and quality has been
observed, including sperm count, ejaculate volume, alterations in
sperm concentration and morphology [2]. Recent studies suggest
that men with abnormal semen parameters have a higher risk of testicular malignancy [3]. Nowadays, environmental and lifestyle
factors could be possible contributors to infertility conditions,
such as use of smoke sigarettes, increasing of both parents age
conception, abuse of alcohol and drugs, physical inactivity, obesity,
social stress, exposure to environmental contaminants (polycyclic
aromatic hydrocarbons-PAHs, or heavy metals, for examples) and
air pollution [4,5]. In particular, epidemiological and experimental
studies explained the link between air pollution and alterations of
sperm parameters as the main risk factors for male infertility.
Human activities such as transport, industrial and agricultural
emission are considered the main causes of air pollution (solid
particles, liquid droplets or gases), and people that living near these
area, are more exposed to henanced emission source of carbon
monoxide (CO), nitrous dioxide (NO2), sulfur dioxide (SO2), ozone
and lead [6]. Ambient air pollution is associated with systemic
increases in oxidative stress, to which sperm are particularly
sensitive. In this contest, reactive oxidative species (ROS) have been
related with a broad array of spermatogenensis effects, including
the decrease of progressive motile sperm count, viability, abnormal
sperm morphology, and fertilization rate and spermatogenic cell
numbers [7]. In Italy, 12,482 areas with a high risk of environmental
pollution, mainly due to industrial emission, were been identified.
Some central provinces, such as Città di Castello, Foligno and Perugia
exceeded the limit set for particulate matter with diameter less than
10 microns (PM10) and O3 emissions, in 2019. In particular, Terni is
one of the most polluted urban and industrial area in Central Italy
[8]. In fact, is situated in an intermountain depression, delimited
by the Apennine mountain range. This area is characterized by the
presence of typical urban PM10 emission sources such as vehicular
traffic, domestic heating and industrial emission sources from a
power plant for waste treatment. Peculiar geomorphological and
meteorological conditions of Terni basin, limit the dispersion and
augment the accumulation of the atmospheric pollutants.
The “Thyssen Krupp AST”, a large steel factory founded at the
end of 19th century and two more recent chemical industrial areas,
are located close to the city center [9]. As a result of the intensive
industrial activities and the geographical location, atmospheric
pollution is the major local issue with high PM concentrations
occurring throughout the year. According to European Commission
Law, the daily maximum PM10 concentration allowed in cities is 50
μg-3 [9]. The threshold has not to exceede more than 35 times per
year. In Terni, the atmospheric PM10 concentration exceede that
daily limit on more than 70 days in 2012, as recorded by Regional
Agency for Environmental Protection (ARPA), Umbria. The aim of
the present study is to provide an association between ambient air
pollution and sperm quality, analyzing seminal biofluid parameters
of man living in the urban area of Terni- Papigno, with a high risk of
pollution, comparated with those who live in rural areas with low
risk of pollution.
Materials and Methods
Study Participants
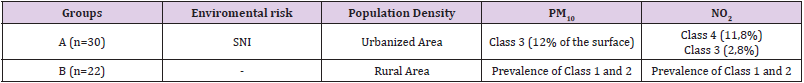
Signed written consent was obtained from all 52 participants (age of 20–40 years) enrolled in this study from January 2018 to December 2019. Patients referred to the Seminology Laboratory of the Division of Andrology and Urology Department for an infertility evaluation. Female partners of the infertile men were subjected to general gynecological evaluation and were reported to have normal reproductive health. Residence in the province of Terni was an inclusion criteria while, systemic and cronic disease, genetic abnormalities, alcohol or drug abuse, hormone treatment, varicocele infection microchidism and cryptorchidism, prostatitis and other factors that could affect semen quality (such as fever, medications, exposure to X rays etc.) were exclusion criteria. Men were divided in group A and B that includes 30 patients from the high pollution environmental risk area of Terni-Papigno and 22 subjects that live in neighboring areas, with a low risk of pollution, respectively (Table 1).
Table 1: Demographic and environmental patient’s classification.
Note: SNI: Sites of National Interest; PM10, Particulate Matter ≤ 10 μm; NO2, Nitrogen dioxide; OCSE Organization for Economic Cooperation and Development, class 1 and 2: rural area with population density <150 inhabitants/ km2 and PM10< 10 μg/m3; class 3 and 4: rural area with population density >150 inhabitants/ km2 and PM10> 10 μg/m3
Semen Analysis and Preparation of Samples
Semen samples were collected at the Andrology and Urology
Laboratory by masturbation into a sterile container after 2–7 days of
sexual abstinence and were analyzed immediately after liquefaction,
according to the WHO guidelines [10]. Each sample was evaluated
for seminal volume, pH, total sperm count, progressive motility,
and morphology and leukocyte concentration. Semen volume was
measured by graduated pipettes. Calibration strips were used
to measure the seminal fluid pH. For the evaluation of the sperm
concentration, following semen liquefaction, 10 μL of non-diluted,
well-mixed semen sample was at first loaded in the middle of a
clean Burker counting chamber, maintained at the temperature of
37 °C, gently covered with a cover glass, and examined using 200×
or 400× magnification. The sample was diluted before proceeding
with the sperm count. 1:2 dilution was used, strictly following the
WHO 2010 manual recommendations [10]. The final concentration
was calculated as: [(number of spermatozoa counted/the number of
lines) x dilution factor] and expressed as 106 spermatozoa/mL. To
evaluate the sperm motility, immediately after semen liquefaction,
10 μL of undiluted, well-mixed semen sample was loaded in the
middle of a clean Neubauer counting chamber, maintained at
the temperature of 37 °C, gently covered with a cover glass, and
examined using 200× magnification.
Sperm motility was assessed in 200 random spermatozoa
and characterized as progressive and non-progressive motility.
The total motility was calculated as the sum of progressive and
non-progressive motility. Both progressive and total motility were
expressed as percentages. Sperm morphology was evaluated in 200
spermatozoa and the value was expressed as percentages. Finally,
the vitality was assessed using eosin staining according to the WHO
recommendations [10].
Statistical Analysis
Data were analyzed with GraphPad Prism 6.0. Results were reported as mean ± standard deviation (SD) Analysis of variance and Kruskal-Wallis tests were performed. The significance threshold was set at 0.05.
Results
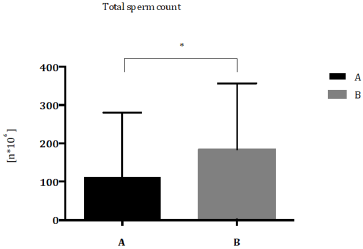
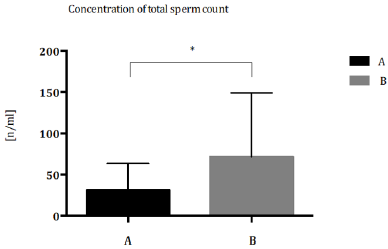
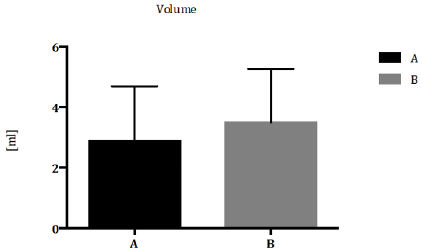
Patients were classified according to Demographic and Environmental characteristics (Table 1) Total sperm count (Figure 1) and sperm concentration (Figure 2) decreased significantly in seminal biofluid of patients living in urbanized area of Terni- Papigno (group A) when compared to subjects who lived in rural areas (group B). Seminal volume (mL) did not reach significant difference (Figure 3) between the groups considered.
Figure 1: Total sperm count (n*106) expressed as median with range and interquartile range in group A compared to group B. *P<0,05.
Figure 2: Concentration of total sperm concentration (n/ml) expressed as median with range and interquartile range in group A compared to group B. *P<0,05.
Figure 3: Seminal volume (ml) expressed as median with range and interquartile range in group A compared to group B. *P<0,05.
Discussion
In this study, conventional semen parameters (volume,
appearance, pH, motility, and viability, and morphology, presence
of aggregations, agglutination and leukocyte concentration) were
analyzed for each sample according to the 2010 WHO criteria. Our
results showed a significant decrease of total sperm count in group
A patients respect to group B subjects (Figure 1). Nowadays, several
studies showed how pollution can affect human fertility. Sperm
count and sperm motility were reduced in men living in polluted
cities. A decrease of sperm count was observed in America and
Northern Europe during the last 50 years [2] and the reduction
of semen quality and sperm count especially in industrialized
countries supports the evidence that adverse environmental
factors are key factors for men infertility [11]. Furthermore,
different studies reported the enhancement of infertility, urogenital
malformation and chronic disease (cancer, diabetes, etc.) in areas
with a high environmental pressure [12]. In group A, a significant
decrease of sperm concentration was demonstrated, respect to
group B patients (Figure 2). In 2019, a longitudinal analysis on
8.945 semen samples, suggested the specific role of O3 pollution
in sperm concentration [13]. The effect of air pollutants and ozone
on sperm quality, was strongly elucidated by Sokol, et al. [14]; they
reported that pollutants have no effect on sperm quality, however,
ozone could make changes in sperms, including sperm DNA
fragmentation via oxidative stress, resulting in decreased fertility
[14].
The toxicity of O3 was largely demonstrated to be the major
oxidant of photochemical smog and its exposure produces reactive
ROS at respiratory level [15,16]. Extra pulmonary toxicity suggests
that O3 or O3 reaction-products can cross blood-gas barrier and be
absorbed into the circulating bloodstream, creating an environment
caring to an inflammatory reaction [17,18]. It is unclear how
O3 can negatively affect sperm quality, but O3-induced oxidative
stress could be a possible mechanism, through which testicular
and sperm function could be altered [19,20]. Under physiologic
conditions, spermatozoa exist in a balanced environment of ROS and
antioxidants, where ROS determine the biochemical steps required
for normal fertilization (capacitation and the acrosome reaction).
However, excessive amounts of ROS produced by leukocytes and
immature spermatozoa can damage mature spermatozoa and the
integrity of sperm DNA [21,22]. Concerning seminal volume (mL),
our results did not reach significant difference between groups
considered (Figure 3). Several studies suggested that alterations
of semen parameters, including volume, progressive motility,
total motility or morphology, may not relate to air pollution [23].
Several works observed a reduction of both sperm motility and
morphology, associated with air heavy metals (lead, mercury and
cadmium) [24,25], that determine alterations in sperm DNA.
Rubes and colleagues showed that short-term exposure
to pollutants caused serious damages in men and women’s
reproductive system. Moreover, this study demonstrated that the
incidence of sperm DNA alterations due to air pollution is higher
in middle-aged men [26]. Also, exposition time of contaminants
seem to be determinant, in fact a study of Hammoud showed that
3 months exposure to air pollution decreases motility levels, while
removing contamination can restore normal parameters levels [27].
In an extensive cross-sectional study, Xu and co-workers, founded a
strong correlation between motility, concentration, and morphology
of semen biofluid of men exposed to air pollutants with the use of
cigarette and alcohol [28]. On the other hand, Selevan, et al. [29]
did not observed any relevant alerations in sperm count, motility
and morphology of young men exposed to air pollutants, except for
sperm DNA and chromatin [29]. Moreover, several authors specify
that the morphological change of spermatozoa after exposure to
pollutants is not an indicative diagnostic parameter. Association
between air pollution and alteration of specific semen parameters
is not clear and different relevant factors, such as geographic areas
and lifestyle, should be considered for male infertility diagnosis.
Conclusion
The current study provides evidence of an association between ambient air pollution and sperm quality. Patient residents in areas with high environment exposure had a significantly decrease in sperm quality especially for sperm concentration and count but had no impact on the other sperm parameters of spermogram (motility, morphology, vitality). The individual role of specific pollutants is difficult to identify, since patient in this study are typically exposed to several pollutants simultaneously. The physiopathology leading to altered fertility is poorly understood. In the literature there are forward four mechanisms to explain the negative impact of air pollution on sperm, as hormonal disturbances, oxidative stress induction (ROS), cell DNA and epigenetic alterations, probably working in combination. Clearly, more research is needed to understand the detrimental effect of the pollutants on sperm and to characterize their action in more detail. Our results suggest the important role of human sperm as an early and sensitive biomarker of environmental pollution as it could represent an ideal tool for investigating and promoting health surveillance especially in environmental risk areas. Environmental contaminants and bad lifestyles can impair reproductive health and overall health, encouraging the development of chronic degenerative diseases affecting the adult and, through the sperm epigenome changes, future generations. Thus, identifying risk factors to improve the management of human wellness and health throughout standardized analysis, which correlates the toxic bioaccumulation of the seminal fluid with the multiple semen parameters, might be the main objective to be considered in public prevention policies.
For more
Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.