Pulsatile Vs Non-Pulsatile Intracranial Blood Flow: Animal Model of Blood Flow Restoration in Brain Tamponade
Introduction
In neurosurgical practice brain tamponade represents the
ultimate limit for treatment. It is defined as a progressive
intracranial
pressure (ICP) increase up to values close to arterial blood pressure
producing a reverberating flow pattern in the cerebral arteries with
no net flow [1-3]. Nowadays, patients reaching such a condition
are labeled as untreatable due to the lack of effective treatment.
Decompressive craniectomy might in peculiar conditions, such as
in very little children, overcome the aforementioned limit thanks to the
incredible capability of a still growing brain to recover from
extensive injuries but that is not the case in adult or elder patient.
Throughout the literature there are several papers addressing
the matter but still no clear advance was proposed. In fact, many
of the papers are still at the animal levels also due to the actual
difficulties in creating an ethically approvable human model. This
aspect is linked to the fact the patients near brain tamponade
conditions have to be rapidly treated whenever possible being
hard to create a double group-controlled study. Furthermore, it is
not so easy to define the actual limit in which brain tamponade
become irreversible. The authors themselves, in previous papers,
highlighted how even in prolonged brain tamponade conditions,
metabolism inside the neuronal cells still continue even after
prolonged ischemia time [4,5].
The idea of overcoming the blockage in cerebral blood flow
modifying its modality derived from a previous report of residual
arterial and venous pulsation even in tamponade brains [1]. In order
to do so, we hereby present an animal model in which changing the
modalities of brain blood supply from pulsatile to continuous it
might be possible to maintain cerebral perfusion even in conditions
of highly elevated intracranial pressure.
Material and Methods
Five male sheeps (30-35 Kg) were sedated using intramuscular
Atropine (0,5-1 mg) and Ketamine (10 mg/Kg). Each animal was
placed supine on the operating table, intubated and anesthetized
with Halothane (0,8-1%) and Pancuronium Bromide (0,5 mg/h
intravenously administrated). These sheeps were evaluated for the
whole duration of the procedure using:
a) Electrocardiogram
b) Systemic arterial bold pressure (measured through a line in
the obturator artery) (SAP)
c) Carotid arterial blood pressure (CAP)
d) Middle cerebral artery blood flow measured using doppler
ultrasound (placed to an ad-hoc craniotomic window)
e) ICP measured using an intra-parenchymal sensor (ICP Express
Codman) placed using a parietal burr hole.
Once sedated each animal was prepared in the following way.
Two inguinal incisions were made to isolate the femoral arteries
that were exposed trough blunt dissection and cannulated.
Similarly, through a neck midline incision the carotid arteries
were found and prepared. In the meanwhile, a hydraulic circuit
was created to ensure extracorporeal circulation. Such a circuit
was composed by sylastic tubes, a peristaltic pump, a three-liter
reservoir placed at 3-meter height from the ground and lastly from
a mechanism granting pulsatility in order to mimic cardiac output.
This mechanism is composed by an electric engine connected to
a piston compressing the elastic portion of the tube exiting the
reservoir. By doing so modifying the compression speed and the
distance of the piston from the tube is possible to modify pulsation
frequency and amplitude. The described circuit has three terminals,
one for each femoral artery and the remaining one for the left
carotid artery (the terminal ends with a Y connector). The whole
circuit is replenished before starting with saline solution added
with 25000 unit of heparin in order to avoid clotting inside it. To
avoid animal hypovolemic state, the reservoir is filled with a liter of
saline solution before starting.
To create a condition of intracranial hypertension saline
solution will be sent into the subdural space using a 20 Gauge
needle inserted through a small, angulated burr hole which is
also sealed with acrylic resin in order not to let the fluid escape
around the needle. Infusion flow speed was regulated according
to the parameter measure by the intra-parenchymal sensor. After
clamping of the brachiocephalic trunk, the circuit can be activated.
Whenever doing so, the blood taken from the femoral arteries is
aspirated and carried into the reservoir from where, thanks to
gravity, it flows into the left carotid artery. Thanks to the Y connector
the blood in the left carotid artery can flow both toward the brain
and towards the base of the brachiocephalic trunk granting blood
supply to the whole brachiocephalic territory. The pulsation
machine intervenes in this setting in order to transform a pulsatile
flow into a continuous one without creating relevant changes in
medium arterial pressure. Once completed animal preparation,
three different experimental conditions were evaluated in order to
measure the cerebral perfusion pressure (CPP=CAP-ICP) value at
which cerebral blood flow (CBF) blockage appear in each of them.
The aforementioned conditions are:
a) Normal condition
b) Continuous laminar flow created using EC
c) Combined model. In this model the brain is submitted
to pulsatile circulation created with the aforementioned pulsatile
machine in EC switching in a second moment to continuous flow in
order to evaluate differential response to flow modifications.
At the end of the experiment the animals were sacrificed being
still under general anesthesia using an intravenous administration
of 10 mEq potassium chloride. The whole experiment was carried
on in accordance with the EU Directive 2010/63/EU for animal
experiments.
Results
A. Model 1: mean CAP value is 110 mmHg (ranging from
100 to 130 mmHg) while mean ICP value is 15 mmHg (ranging
from 12 to 18 mmHg) and mean MCA speed is about 10 cm/sec.
Starting saline subdural infusion, ICP value start increasing while
CBF progressively decrease. This process continues until ICP reaches 70 mmHg with consequential CBF blockage. Even though
no blood flow can be measured at this moment, CPP is still present
and greater than 40 mmHg. At the same moment, a different
behavior of CBF velocity can be observed. In fact, even if CPP is still
present flow velocity reaches zero concomitantly wit tamponade.
Both observations tend to recover baseline condition once stopped
infusion.
B. Model 2: the initial increase in ICP and decrease in CBF
speed is the same of model 1 but, unlike with pulsatile flow, CBF
arrest is reached with higher ICP value. In fact, ICP values similar
to CAP are needed in this case with a residual CPP of 15-16 mmHg
to observe cerebral tamponade. The observation concerning CBF
speed overlaps what seen in model 1. As in model 1 this condition
is reversible after infusion arrest.
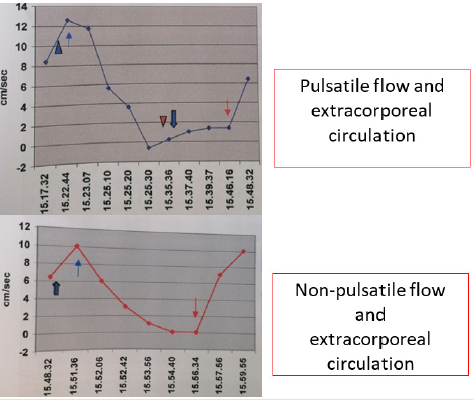
C. Model 3: the combined model shows firstly how normal
cardiac circulation can be achieved using pulsatile EC with similar
results on CPP and CBF speed. On the other hand, it shows how,
switching from pulsatile to continuous flow in absence of relevant
changes in CPP, a gradual and stable intracranial circulation
can be obtained as documented by doppler ultrasound. The
aforementioned results are summarized in Figure 1.
Discussion
Throughout the literature, there are very few reports regarding
flow typology in intracranial circulation. Such papers are mostly
related to intracranial changes after ischemic heart failure.
Reviewing the literature trying to select the most fitting papers,
only two authors slightly address the problem. the first one only
mentions non pulsatile blood flow as something unclear as well as
a potential sign for proximal arterial occlusion [6], while the other
one, suggests the importance of pulsatile flow during reperfusion
without addressing at all flow modifications during tamponade
[7]. To overcome such a lack of evidence on the matter, the authors
devised the presented experiment. The aim was to analyze whether
changing cerebral blood flow from pulsatile to non-pulsatile was
possible to overcome brain tamponade. Such an experiment
was founded on the idea that the very “normal” blood pulsation
coupled with Starling resistor functioning is at the base of cerebral
tamponade. Physical laws states that flow is driven by the presence
of a pressure gradient between two compartments connected by
a channel. Thus, as long there is a gradient there will be flow, no
matter how small the caliber of the channel will become. Flow stops
then after the closure of the channel or after disappearance of the
gradient. The application of such physical law to the intracranial
system were evaluated for the first time by Chopp et al. who created
a model simulating the intracranial space and its modifications
during infusion tests [8].
In order to describe what happen in normal conditions, it is
important to remember that intracranial circulation is pulsatile
and that pressure wave propagation speed inside the vascular
system is slower than the liquoral one due to the resistance in
capillaries and veins. Thus, whenever there is an increase in intracranial pressure, the aforementioned difference in transient
propagation speed lead to an early closure of the veins and of the
Starling resistor before intravasal pressure could match outer one
maintaining positive flow. When the vein walls contact each other
the possibility to re-open is lost leading to tamponade. On the
other hand, if the circulation were non-pulsatile a net flow would
be always present thanks to the persistence of pressure gradient.
Such persistence is granted by the absence of a pulsation wave
preventing the previously described vein closure mechanism. The
channels will become smaller in an asymptotic way never actually
closing and preventing the reach of zero net flow. Obviously, this
situation is theoretical and in reality, the channels will eventually
close, but a greater intracranial pressure would be needed. In order
to demonstrate such an assumption, we have created a model of
selective extracorporeal brachiocephalic circulation in order to
send laminar flow to the brain without affecting body circulation.
The selection of the sheep as animal model was made in order
to simplify the experiment having this animal a peculiar anatomy of
the brachiocephalic trunk. In fact, in this setting all of the vessels,
emerging in the human from the aortic arch, start from this trunk.
From left to right it emerges first the left subclavian artery the
two carotid arteries and last the right subclavian artery. Such
conformation simplifies the experiment granting the selectivity
control of the cerebral blood flow through the manipulation of
a single vessel. Nonetheless, it is important to remember that
collateral circulation might be present in selected cases reducing the
power of the experimental model. In the sheep model though, such
collaterals disperse most of their contribution to the spinal roots
and to the neck muscle making the amount of cerebral distribution
negligible. Dividing the experiment into three moments granted
us the possibility not to miss biases in the model. In model 1 the
authors confirmed a similar trend between sheeps and humans
regarding brain tamponade. Blood flow ceases concomitantly with
an increase of ICP over CPP reaching brain tamponade even in
condition of persistent low CPP. Model 2 differs from model 1 in the
need for a higher ICP value to reach tamponade and flow absence.
Such a finding suggests a higher threshold to be reached in order
to cause it. Finally, model 3 unites the previous ones and improves
them showing how a change in flow type might overcome a preexisting
tamponade situation offering a possible novel treatment
strategy. The most striking data reside in the reappearance of blood
flow during tamponade after the change from pulsatile flow to
continuous one.
Conclusion
Brain tamponade in neurosurgery represents nowadays the terminal line for treatment. Every effort has to be made in order to find a way to overcome such a limit. Our data might represent the first step in that direction showing how changing cerebral flow even tamponade can temporarily overcome. Even though this is only an animal experiment it might open the way to further animal experiment and thus to human ones.
For more
Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.