Source-Induced Dissociation Vs Collision-Induced Dissociation Fragmentation Behavior of Four Antiviral Drugs in a Liquid Chromatography Ion-Trap Mass Spectrometry
Introduction
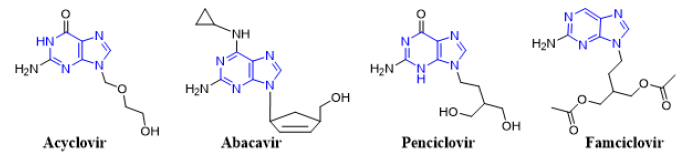
Mass spectrometry (MS) is an important tool for analyzing or detecting a wide range of organic, inorganic, natural, unnatural molecules, especially drugs, proteins, peptides are of interests beside routine analysis [1]. MS measures the mass-to-charge ratios of ions of an ionized sample, which may be gas, liquid or solids. Mass-to-charge ratios may obtain for a charged molecule or charged fragments. MS has both quantitative and qualitative uses. Numerous ionization methods are used in MS experiments, such as chemical ionization (CI), matrix assisted laser desorption ionization (MALDI), electron impact (EI), atmospheric pressure chemical ionization (APCI), and electrospray ionization (ESI). ESIMS has been applied to the analysis of oligonucleotides [2, 3] drugs and drug metabolites [4] oligosaccharides [5, 6] environmental contaminants [7, 8] glycoproteins [9] and many other types of compounds [10-15]. In atmospheric pressure ion sources, e.g., ESI or APCI, dissociation of ions can occur inside the ionization source (source induced dissociation; SID) before reaching the mass analyzer. SID has been used by several research groups [16-22]. Usually, SID is used to analyze single mass, whereas CID is used to analyze fragmentation. Both SID and CID techniques having advantages and disadvantages [23, 24]. While CID technique is very much effective to analyze pure sample with much more information about structural data [25]. Acyclovir, [26, 27] abacavir, [28, 29] famciclovir [30-33] and penciclovir [10] are the known antiviral medication used to prevent viral diseases. All of these antiviral drugs having common purine moiety [34] (Figure 1) and 9-position of purine are substituted with deferent alkyl chains. Mass spectrometric study of these structurally similar compounds may reveal lots of information for further analysis process during antiviral doses. As a medicinal chemist, it is our duty to develop drug molecules / administration procedures / toxicity evaluation process and / identification in a mixture or in biological matrixes. Therefore, there is always a room for extending such development using modern scientific methods such as mass spectrometry.
Figure 1: Chemical structures of antiviral acyclovir, abacavir, penciclovir and famciclovir showing a common purine nucleus.
Here, analysis of four antiviral drugs acyclovir, abacavir, penciclovir and famciclovir serves to figure out how to extend the qualitative power of ITMS and to maximize the benefit from this mass analyzer. These four drugs also were selected as they contain a common purine nucleus in their chemical structures which might generates a common fragmentation pattern. Above mentioned four known antiviral drugs were studied for their fragmentation pathways and data of SID and CID of ESI IT were compared. The detail fragmentation pathways of all ions observed in the in-source fragmentation spectrum of compounds were elucidated by further dissociation of each of these fragment ions using MS2, MS3 and MS4 fragmentation stages.
Experimental
An Agilent 6320 Ion-Trap connected (LC-MSn) mass spectrometer was used. Compounds were dissolved in DMSO and used as standard solution, then diluted with a mixture of HPLC grade water and acetonitrile (1:1). Fragmentation pattern of these four antiviral drugs by direct injection using infusion pump were performed. 10 μL of each sample was injected with direct injection, flow rate was 0.4 ml/min., run time was set to 5 minutes, drying gas was N2 (flow was 12 L/min), temperature was set to 350 °C, nebulizer pressure was 50 psi, smart target was 10,000, accumulation time 150 ms, scan range was 20- 400Da and Ion source was ESI, in positive mode with ultra-scan. There are several factors that can influence the fragmentation spectra, such as the collision energy, major precursor ion, electrospray mode (positive or negative) and capillary exit voltage. In IT, product ion scan was performed before the in-source fragmentation to determine each compound’s related fragment ions, the capillary exit voltage was optimized to produce adequate in-source fragmentation. The data compared to multistage fragmentation using CID. We made all default parameters except capillary exit voltage which was 100 V for CID and 200 V for SID.
Results and Discussion
CID MSn Fragmentation Pattern of Abacavir
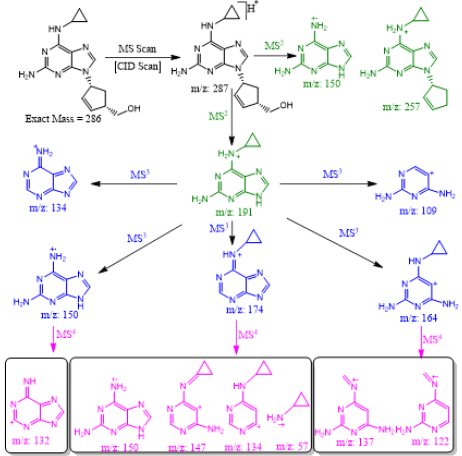
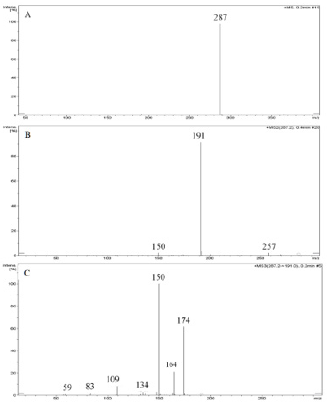
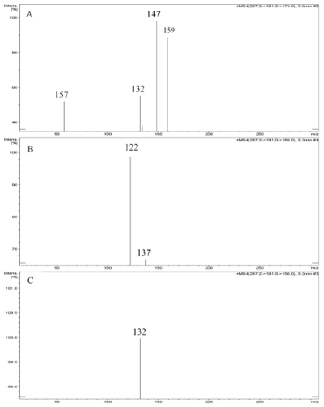
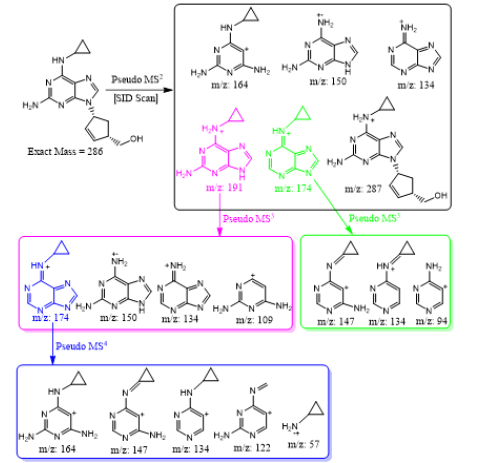
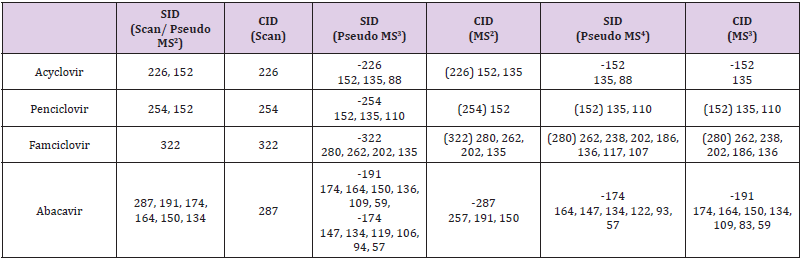
Abacavir was set for an example of comparing two fragmentation techniques. As shown in (Figures 2 & 3), full scan of abacavir gave m/z 287 (Figure 2A) and MS2 of abacavir gave three fragments at m/z 257, 191 and 150 (Figure 2B). Among three fragments, m/z 191 was a prominent peak which was further bombarded (MS3) and gave m/z 174, 164, 150, 134, 109, 83 and 59 (Figure 2C). Consequently, high intense three ions m/z 174, 164 and 150 were further bombarded (MS4) in which, m/z 174 gave four ions at m/z 159, 148, 132 and 157 (Figure 3A), m/z 164 gave two ions m/z 137 and 122 (Figure 3B), and m/z 150 gave a single ion at m/z 132 (Figure 3C).Based on (Figures 2& 3), we have drawn Scheme 1 for the fragmentation pattern of abacavir in CID mode. As shown in scheme 1, after four steps (MS4) fragmentation, abacavir gave maximum fragments at m/z 150, 147, 137, 134, 132, 122, and 57. SID pseudo-MSn fragmentation pattern of abacavir. As shown (Figure 4), pseudo MS2 of abacavir gave m/z 287, 191, 174, 164, 150 and 134 (Figure 4A), and pseudo MS3 of major two fragments, m/z 191 and 174 was further bombarded (MS3) and obtained m/z 191 gave m/z 174, 164, 150, 136, 109 and 59 (Figure 4B) and m/z 174 gave m/z 147, 134, 119, 106, 94 and 57 (Figure 4C), respectively. Pseudo MS4 of high intense m/z 174 gave m/z 164, 147, 134, 122, 93 and 57 (Figure 4D).
Figure 2: CID MSn fragmentation pattern of abacavir:
A. MS scan for abacavir,
B. MS2 of 287 and
C. MS3 of 191.
Figure 3: CID MS4 fragmentation of three ions (174, 164 and 150):
A. MS4 scan of 174
B. MS4 scan of 164 and
C. MS4 scan of 150
Figure 4: SID pseudo-MSn fragmentation pattern of abacavir:
A. MS scan for abacavir applying capillary exit voltage,
B. product ion for 191
C. product ion for 174 and
D. MS3 for 174.
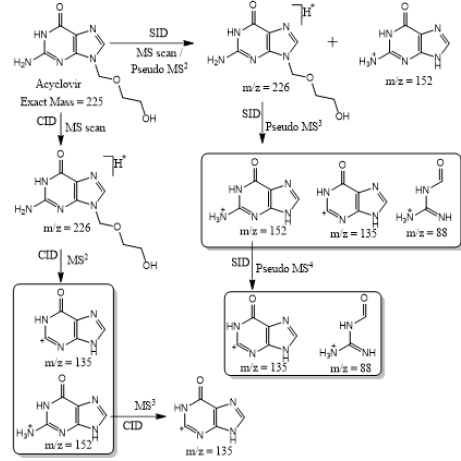
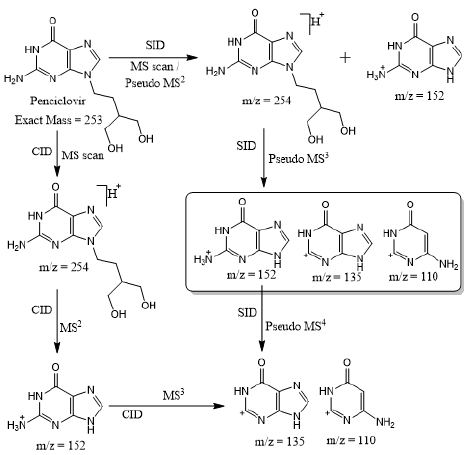
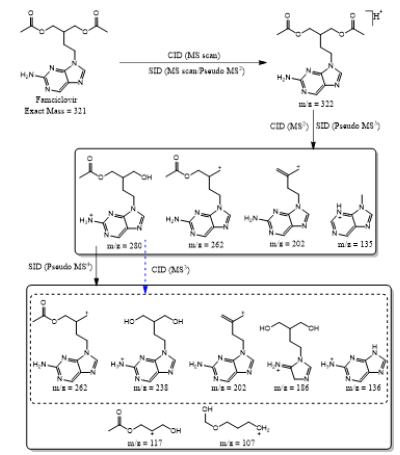
Based on (Figure 4), we have drawn Scheme 2 for the fragmentation pattern of abacavir in SID mode. As shown in scheme 2, in two steps (pseudo MS3) fragmentation, abacavir gave maximum fragments at m/z 174, 150, 147, 134, 132, 109 and 94 and pseudo MS4 of m/z 174 gave five fragments at m/z 164, 147, 134, 121 and 57. SID and CID of acyclovir, penciclovir and famciclovir. Other three antiviral drugs acyclovir, penciclovir and famciclovir mass spectrometric study in SID and CID results are summarized in Table 1 along with abacavir. As shown in Table 1 and depicted in Scheme 3, SID scan of acyclovir gave m/z 226 [M+H] + with 152 and MS2 of main peak m/z 226 gave three main fragment at m/z 152, 135 and 88. Among three fragments, m/z 152 was further bombarded and obtained m/z 135 and 88. On the other hand, in CID scan, acyclovir gave m/z = 226 [M+H] + only. Which was further bombarded for MS2 and obtained m/z 152 and 135 only. MS3 of m/z 152 gave only one fragment from SID at m/z 135.As shown in Table 1 and depicted in Scheme 4, SID scan of penciclovir gave m/z 254 [M+H] + with 152 and MS2 of main peak m/z 254 gave three main fragment at m/z 152, 135 and 110. Among three fragments, m/z 152 was further bombarded and obtained m/z 135 and 110. On the other hand, in CID scan, penciclovir gave m/z = 254 [M+H] + only. Which was further bombarded for MS2 and obtained m/z 152 only. MS3 of m/z 152 gave same fragments as we obtained from SID, which is m/z 135 and 110. As shown in Table 1 and depicted in Scheme 5, SID and CID scan of famciclovir gave only protonated peak at m/z 322 [M+H] +. MS2 of this m/z 322 also gave four same fragments at m/z 280, 262, 202 and 135. Among those peaks, a main peak m/z 280 was further bombarded and obtained seven fragments m/z 262, 238, 202, 186, 136, 117 and 107 in SID and 5 fragments m/z 262, 238, 202, 186 and 136 in CID. Common fragmentation paths of abacavir, acyclovir, penciclovir and famciclovir.
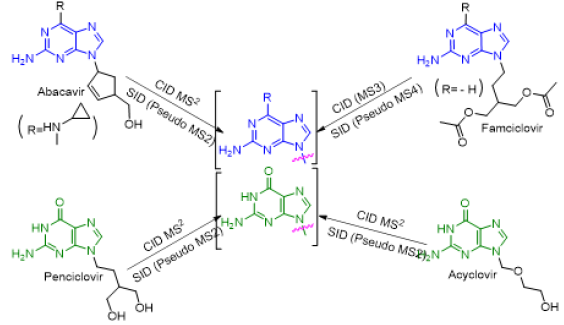
As depicted in Scheme 6, all four antiviral drugs gave similar fragmentation pathway. Abacavir, acyclovir and penciclovir gave purine moiety while SID / CID MS2 was applied, on the other hand, famciclovir gave similar pathway with purine moiety while SID (Pseudo MS4) / CID (MS3) was applied (Scheme 6).
Scheme 6: Fragmentation pathways of all antiviral drugs (abacavir, acyclovir, penciclovir and famciclovir).
Conclusion
Excellent qualitative information was obtained using sourceinduced dissociation (SID) comparing to collision-induced dissociation (CID) in an ITMS. Fragmentation pathways of four antiviral drugs abacavir, acyclovir, penciclovir and famciclovir gave similar information regardless fragmentation method as they contain purine nucleus in their building blocks. This method can be applied for the detection /identification of know/unknown drugs or their metabolites in-vitro / in-vivo or in biological matrixes by comparing their fragmentation pattern and using different possible fragmentation tools to maximize the benefit of ITMS in the process of drug development.
For more
Articles on : https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.