Effect of Physical Exercise on Cardio Metabolic Risk in Mexican Adolescents with Metabolic Syndrome
Short Communication
Introduction
The metabolic syndrome (MetS) is a set of dysfunctions and disorders that increase the risk of developing chronic diseases mainly of cardiovascular and metabolic type, such as diabetes mellitus, coronary disease, stroke and others that currently represent the main causes of morbimortality in Mexico [1]. Although MetS is more prevalent in the adult population, recently an increase in its incidence has been observed in children and adolescents, which is worrisome due to the health risks this represents. Some authors believe that increase in the incidence of MetS in children and adolescents is due to the high prevalence of obesity, excessive caloric intake and sedentary lifestyles [2]. At world level, Mexico occupies the first places of obesity in pediatric population, therefore it is estimated MetS represent a public health problem in children and adolescents [3]. The diagnosis of MetS in adolescents includes elevated blood pressure levels, increased waist circumference, increased triglyceride levels and fasting glycaemia, as well as decreased HDL cholesterol [4]. There are several guidelines for diagnosis MetS in adolescents. The diagnosis must be adjusted with age and sex [5], since adolescence is a period of rapid growth, in addition to the dimorphic effect of sex hormones on fat tissue [6]. Cardiometabolic risk is the likelihood that a person will have cardiovascular damage, it can be determined with different mathematical models based on the numbers of certain metabolites such as insulin, lipid profile, glycemia and others.
Among the main indicators of cardiometabolic risk are: insulin resistance or insensitivity, pancreatic beta cell dysfunction and atherogenic risk, which represent a poor prognosis for health [7,8]. MetS can be treated with different therapeutic approaches, such as lifestyle modification, pharmacological treatments and even surgical interventions [9]. Physical exercise is one of the strategies that have been successfully used to manage MetS and some other metabolic diseases such as diabetes mellitus and obesity [10]. Some publications have reported that exercise reverses MetS [11,12] having therapeutic effects on its components, however others have found no effects on MetS, but they report benefits in other metabolites such as adipokines and other inflammatory markers [13,14]. This controversy may be related to the type and duration of exercise. Physical exercise is an accessible activity for most people, it is economic, and it can also have a recreational component, which tends to be attractive to adolescents and to improve adherence to treatment, which is one of the keys to therapeutic success [15]. In Mexico, some studies have estimated that the highest prevalence of MetS in adolescents is found in the southeast of the country, probably due to the high rates of obesity and overweight in this same region [16,17] and make it necessary to implement therapeutic strategies to improve health in this population group.
The goal of the present study was to analyze the effect that moderate intensity physical exercise for 12 weeks has on components of MetS and cardiometabolic risk in adolescents in southeastern Mexico.
Methodology
Quasi-experimental study was conducted on adolescents with MetS in Merida, Yucatan, Mexico. MetS components and cardiometabolic risks were analyzed before and after physical exercise model. Sample selection. Adolescents aged 15 to 18 registered in high schools were invited to participate in the study. Was a non-probabilistic sample of 313 adolescents that accepted to participate through the letters of assent and informed consent? After first evaluation, 46 met MetS criteria of which 86% complete de exercise model. MetS diagnosis. Based on the criteria of the Latin American Diabetes Association4 (ALAD), which considers presenting at least three of the five indicators proposed by the International Diabetes Federation (IDF) adapted to the adolescent population: Abnormal fasting glycemia (100-126 mg/dl), hypolipoproteinemia [cholesterol-HDL ≤40 mg/dl (men) and ≤50 mg/dl (women)], hypertriglyceridemia (≥150 mg/dl), hypertension [systolic blood pressure (SBP) and /or diastolic blood pressure (DBP) ≥percentile 95] and abdominal obesity [waist circumference (WC) ≥percentile 90]. Anthropometric and clinical measurements. Carried out with fasting of 10-12 hours. Weight (Kg) and body fat percentage was determined with a TANITA Onrom® TBF 300GS body composition scale. Size was determined with the SECA® stadiometer. Body max index (BMI), was established by the formula height (m2)/weight (Kg).
We classified as excess weight those values equal to or greater than the 85th percentile that include overweight and obesity, according to the Official Mexican Standard NOM-047-SSA2-2015 [18]. Body fat was classified as normal or excess, considering age and sex, according to the cut-off points for pediatric patients mentioned by McCarthy et al. [19] Waist circumference was determined with SECA® brand tape at the navel level at the end of a normal exhalation and was expressed in centimeters [4]. Blood pressure was measured with an Omron® brand HEM-7200 digital baumanometer, hypertension was diagnosed when SBP or DPB figures ≥ 95th percentile, in three different measurements. The mean arterial pressure (MBP) was calculated according to the formula SBP-DBP/3+DBP [20]. Biochemical analysis. Performed on venous blood samples, after 10-12 hours of fasting. Glucose and lipid profile measurements were performed in the automated Dimension Chemistry System, Dade Behring®. Serum insulin was determined by immunoadsorption techniques in the Beckman Coulter® equipment, exposed in μU/ml. Hyperinsulinaemia was established with values of 23 μU/ml or < according to the reference standards of the clinical analysis laboratory. The handling of biological residues was in accordance with the Mexican Technical Norm NOM-087-ECOL-SSA1-2002.
Cardiometabolic Risks Indexes. HOMA-IR Index (homeostasis model assessment). Fasting plasma insulin value (μU/mL) x fasting plasma glucose (mg/dL) / 22.5. HOMA-RI in men > 2.29 and in women > 2.21 [21]. QUICKI (quantitative insulin sensitivity check index) is a variation of the HOMA equation, which transforms the data by taking the logarithm and the reciprocal of the glucoseinsulin product, according to the formula [1/ (log insulin + log glucose)]. The values used for RI QUICKI were 0.382 ± 0.007 for nonobese and 0.331 ± 0.010 for obese.7 HOMA Index B. Represents the HOMA of the pancreatic beta cell function and was determined with the following formula: 20 [fasting insulin (μU/ml)/fasting glucose (mmol/ml)-3.5]. The cut-off point of decreased beta cell function was less than 150% [22]. HOMA-S Index. Represents the HOMA model of insulin sensitivity and is used to provide information about glucose metabolism, this value was calculated as 1 HOMA IR x 100. Values > 54.1 for women and > 46.1 for men indicated insulin insensitivity [23]. Triglyceride/glucose index is an indicator of insulin resistance which was determined with the natural log of triglycerides (mg/dL) x glucose (mg/dL)/2. The criteria used was 8.8 for men and 8.7 for women [8] Indicator triglycerides/HDL-c was calculated with the ratio triglycerides (mg/dL)/HDL-c(mg/ dL), the limit value was 3.1 for men and 2.2 for women.8 Plasma atherogenic index was calculated using the log(TG/HDL-c) formula. Values 0.11 or > were considered to be at risk [24]. Cardiovascular atherogenic index was determined with the HOMA-IR x TG/HDLc formula. Value > 28 is considered to be at risk.
Exercise model. Consisted of moderate intensity aerobic exercise (walking, running, jumping and stretching) three times a week for 3 months. The sessions were held outdoors in the morning, lasting 30 minutes. All practices were indicated and supervised. Only 36 participants completed the exercise model. The study was conducted in accordance with the ethical provisions and recommendations issued in the Declaration of Helsinki of the World Medical Association, as well as the Regulations on Research of the General Health Law of Mexico NOM-012-SSA3-2012. The protocol was approved by the Local Committee of Ethics and Research of the Mexican Institute of Social Security (IMSS), registration R-2018- 3203-038.
Statistics
The statistical analysis included averages, standard deviations, medians and ranges. The normality of the variables was established with Shapiro-Wilk test. The components of the MetS and the cardiometabolic risk indexes were expressed as percentages. The difference (Δ) between the initial and final quantitative measurements of each group determined. t-Student tests were used for compared normal data. U-Mann Whitney test were used in non-normal data. Analysis of nominal data were performed with chi-square tests or Fisher’s exact test. SPSS Version 23 software was used (IBM Corp., Armonk, NY, USA). The P-Values ≤.05 were considered statistically significant.
Results
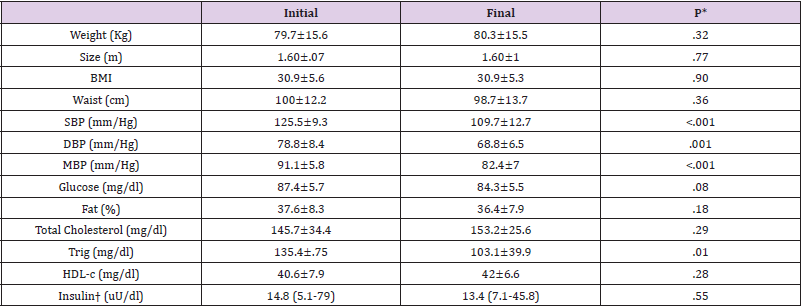
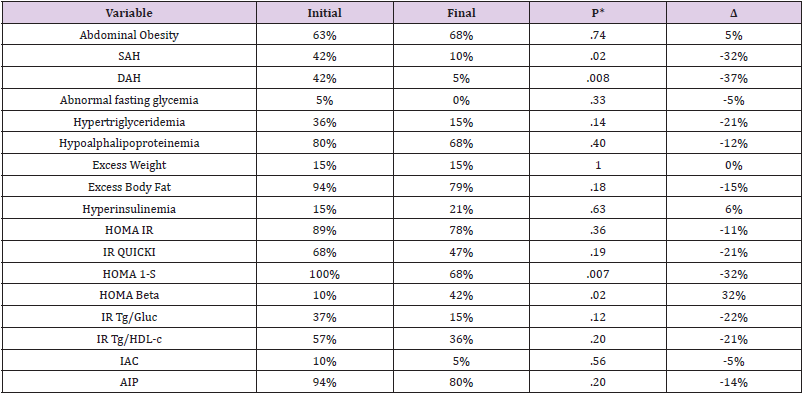
MetS prevalence on the population was 14.69%, with average age of 15.4 ±.6 years. (Table 1) describes the initial and final values of the clinical, anthropometric and laboratory measurements, and it´s comparison. Physical exercise reduced all blood pressure indicators (SBP, DBP and MBP) and triglyceride levels as shown in (Table 1). Based on the cut-off points of the different measurements mentioned in the methodology, the components of MetS, some metabolic dysfunctions and the cardiometabolic risks indexes was determined. The percentages and variation (Δ) between initial and final measures are expressed in (Table 2). According to the order of frequency, the profile of MetS on Mexican adolescent was hypoaliproteinemia, abdominal obesity, hypertension, hypertriglyceridemia, and abnormal fasting glycemia; their percentages are shown in (Table 2). Physical exercise model reduced arterial hypertension (SBP and DBP) and increased insulin sensitivity and pancreatic beta function. Median of MetS components initially was tree and at the end of the intervention was zero (p<0.0001, Wilcoxon, data no shown), and 34 adolescents (84%) recovered from MetS.
Table 1: Anthropometric, clinical, and laboratory values of Mexican adolescents with MetS, after and before 12 weeks physical exercise.
Note: MetS, metabolic syndrome; %, percentage; cm, centimeter; BMI, body mass index; mm/Hg, millimeters of mercury; SBP, systolic blood pressure, DBP, diastolic blood pressure; MBP, mean blood pressure. Col, Cholesterol; Trig, Triglycerides; HDL-c, High Density Cholesterol; mean ± standard deviation; † median (minimum-maximum). t-Student related samples, †U Mann-Whitney
Table 2: Cardio-metabolic risk and other dysfunctions in Mexican adolescents with MetS, before and after 12 weeks physical exercise.
Note: MetS Metabolic syndrome. SAH, systolic arterial hypertension, DAH, diastolic arterial hypertension; %= percentage; Δ percentage of variation= initial value-final value; HOMA-IR, mathematical model of insulin resistance; QUICKI, quantitative index of insulin sensitivity; HOMA 1-S, index of insulin sensitivity; trig, triglycerides; glucose; HDL-c, High density cholesterol; RI, insulin resistance; IAC, cardiovascular atherogenic index. AIP, plasma atherogenic index. Statistical, square chi or Fisher test.
Discussion
The present study focused on evaluating the effect of physical exercise in adolescents with MetS and their cardiometabolic risk. The reduced sample size facilitated exercise monitoring, practiced under the same conditions. We found a prevalence of MetS 15% in adolescents between 15 and 18 years of age, following the diagnostic criteria of the ALAD. Ramirez, et al. [25,26], point out that this classification is ideal for Mexican adolescents. It is very likely that using other criteria such as the IDF or the ATPIII, the prevalence is higher since the use more general MetS diagnostic criteria. The profile of MetS found in the Mexican adolescents was hyperalphalipoproteinemia, abdominal obesity, hypertension, hypertriglyceridemia and abnormal fasting glycemia. In young adults in Brazil, the profile of MetS was hypoalpha- lipoproteinemia, hypertriglyceridemia and hypertension; [27] in Peruvian adolescent’s hyperalphalipoproteinemia, hypertriglyceridemia and abdominal obesity were found [28], both studies indicate that abnormal fasting glycemia was the least frequent component. We found similarities between these profiles, highlighting hyperalphalipoproteinemia as the most frequent component of MetS. Atherogenic dyslipidemia is made up of hypoalphalipoproteinemia and hypertriglyceridemia, which is worrisome because of its pathogenic implications. The frequency of dyslipidemias found by Sapunar, et al. [24] in Chilean adolescents was 38% and by Pajuelo, et al. [29] in Peruvian adolescents with obesity and IR was 46%.
We found 80% of hypoalphalipoproteinemia and 30% hypertriglyceridemia. Dyslipidemias are related to high fat diets, obesity and physical inactivity. [24] It is likely that the gastronomy southeast region of Mexico, being high in saturated and unsaturated fats, is a contributor to the high incidence of dyslipidemias. A limitation of the study was not to analyze the diet of the adolescents, although they were asked to maintain their usual diet to avoid interference with results. Genetics is a factor that can alter the metabolism of fats, differences have been found in the lipid profile between ethnic groups and races, which have been associated with genetic polymorphisms or epigenetic characteristics [29]. Gonzales, et al. [30] in children form this region found polymorphisms associated with obesity and overweight, which may be related to dyslipidemia and MetS. Another factor alters the lipid profile is IR, whose effect at the hepatic level is to increase the synthesis of very low-density lipoproteins, increase the release of low-density cholesterol lipoproteins and triglycerides in the blood, and can also decrease HDL-c by increasing the speed of its degradation and by the abnormal maturation of apolipoprotein 1/HDL-c [21]. Although the gold standard for IR is the euglycemic clamp, this tool was not accessible, so the IR was determined by insulin values. With these methods we found 68-89% of IR in adolescents with MetS.
Using lipid values to determine the IR prevalence in adolescents with MetS was 37-57%, which seems to be more reliable but less sensitive, and may be a more accessible alternative to identify IR in the first level of health care since serum insulin tends to be more expensive and less accessible [31]. Interestingly, during adolescence, which is a period of growth, somatotropin modifies glucose metabolism and produces compensatory hyperinsulinism. This occurs in stage IV and V adolescents according to the Tanner scale [6,21] which corresponds by age to the adolescents studied. Excess body fat was found in 94% of adolescents with MetS. We found excess fat, hyperinsulinemia and insulin sensitivity, subclinical alterations that can favor the development of cardiometabolic diseases [32]. Some adolescents without abdominal obesity presented excess body fat, which may be indicating visceral obesity, some authors have called these people as TOFI (Thin Outside, Fat Inside), which is changing the paradigm of obesity and highlights the need for other tools to identify pathogenic adiposity, since fat affects the metabolism of lipids and carbohydrates and therefore may increase the frequency of MetS and cardiometabolic risk [33]. Exercise decreased blood pressure levels and triglyceride levels in adolescents with MetS. In accordance with these results, different studies have found that the main effect of exercise is the reduction of blood pressure; it seems that muscle activity improves circulation and favors the production of nitric oxide, produces vasodilation which lowers blood pressure.
Nitric oxide may also decrease oxidative stress and improve insulin function in the muscle cell, which represents protective effects at the cardiovascular level [34]. Other authors have also found that physical exercise reduces triglyceride levels in children and adolescents, proposing that the energy spent on exercise comes from the hydrolysis of blood triglycerides and muscle deposits [35]. Meta-analyses have concluded that exercise decreases body fat, both visceral and abdominal, especially when it is of high intensity [36]. Exercise increased beta-pancreatic cell function, this indicator must be correlated with insulin resistance to give a clinical prognosis [37]. In obese adolescents in Taiwan, an intervention similar to ours resulted in decreased body fat, increased insulin sensitivity, and improved b-pancreatic function [38]. The atherogenic index of plasma, a risk factor for coronary disease, was elevated in adolescents with MetS. This risk was high, probably because of diet. González, et al. [39] in European adolescents propose that a diet rich in saturated and hyperglycemic fats increase cardiovascular risk. Adolescence represents a key period where it is possible to adopt healthy behaviors and adolescents with MetS should be made aware of the importance of lifestyle, as well as encourages physical exercise as a habit that can improve cardiometabolic health and prevent the development of chronic diseases in adulthood.
Conclusion
In Mexican adolescents with MetS, physical exercise lowers blood pressure levels, triglycerides and improved some cardiovascular risk indicators such as insulin sensitivity and pancreatic beta cell function. We propose that by increasing the intervention time with physical exercise greater therapeutic effects would be achieved.
| For more Articles on : https://biomedres01.blogspot.com/ |



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.