The Effects of Three Types of Blue Honeysuckle (BH) Preparations on the Uterus in Ovariectomized DDY Mice
Introduction
Climacteric corresponds to the period of life during which women gradually lose their reproductive capacity as a result of aging [1]. This period, according to the World Health Organization, commonly occurs between the ages of 40 and 65. It is closely related to loss of activity of ovarian follicles, with consequent estrogen deficiency [2]. Approximately 70% of women report some type of symptom during the climacteric period. In general, these symptoms are attributed to estrogen deprivation. The most common complaints are vasomotor symptoms and night sweats. In addition, vaginal dryness, dyspareunia, and urinary urgency, which are associated with urogenital atrophy, may interfere with the sex life and quality of life of postmenopausal women [2,3]. The ddY mouse is a closed colony albino mouse. From non-inbred dd mice of the Institute of Infectious Diseases, University of Tokyo, strain 1953 was selected [4] as the most suitable among 6 different strains and was tested for the culture of Clonorchis sinensis [5]. Strain 1953 is now widely distributed and is one of the most widely used inbred mouse strains in Japan and other countries. This strain is generally used in the induction of osteoporosis by ovariectomy [6] and/or sciatic neurectomy [7]. In case of OVX mice, ddY mice have been firstly choice in OVX rodent models [6,8,9]. Phytoestrogens are substances with chemical structure and function similar to that of estrogens and have been shown to bind to estrogen receptors (ER) due to the presence of a phenolic ring [10-12].
Coumestrol and the isoflavonoids genistein, daidzein, and their plant precursors, are mainly found in soybeans and clover [13]. Isoflavones, especially those derived from plants, have various biological activities, and can improve the metabolic symptoms [14] and bone-protective effects [15] of menopause. Phytoestrogen behaves as an estrogen mimic [16], and these estrogenic effects in biological systems believed to relate its structural similarity to estrogen, isoflavonoids exert a weak estrogenic-like effect by binding to both ER-α and ER-β in various tissues [16-18]. Blue honeysuckle (Berries of Lonicera caerulea L., Caprifoliaceae; BH) is a traditional shrub used in folk medicine in northern Russia, China, and Japan, but its fruits are little known as edible berries in North America and Europe, and also in Korea. The berries are a rich source of ascorbic acid and phenolic components, particularly anthocyanins, flavonoids and low molecular weight phenolic acids [19]. These compounds have been reported to have multiple biological activities including strong antioxidant activity [20]. Especially BH extracts have been showed the strongest antioxidant potent among 12 types of colored berries [21], and phenolic rich extract of BH has been shown to possess anti-inflammatory and wound-healing effects in vitro and in vivo [22] and skin protective effects to ultraviolet-induced damages with less toxicity [23]. We studied many anti-climacterium effects of BH and in the present study, the uterus were evaluated by three different types of BHj (juice extracts), BHa (aqueous extracts) and BHm (mixed drink extracts) using bilateral ovariectomized (OVX) female ddY mice.
Materials and Methods
Animals and Husbandry
Total 150 virgin female specific pathogen-free (SPF) outbredmice, Kwl:ddY mice (6-week-old upon receipt; Kiwa Laboratory Animal, Wakayama, Japan) were used after acclimatization for 10 days. Animals were allocated four per polycarbonate cage in a temperature (20-25℃) and humidity (45-55%) controlled room. Light : dark cycle was 12 h : 12 h and feed (Samyang, Seoul, Korea) and water were supplied free to access. One hundred forty mice were used as OVX-induced osteoporotic mice and remainder ten mice were used as sham operation in this experiment. At 27 days after OVX operation, eight mice in each group were selected based on the body weight deviations (32.221.09 g of OVX mice, ranged in 30.10~34.00 g; 28.980.95 g of sham-operated mice, ranged in 27.60~30.20 g, respectively) as follows. All laboratory animals were treated according to the national regulations of the usage and welfare of laboratory animals and approved by the Institutional Animal Care and Use Committee in Daegu Haany University (Gyeongsan, Gyeongbuk, Korea) prior to animal experiment (Approval No. DHU2015-075).
Preparation and Administration of Test Substances
All three types of BH preparations (BHj, BHa and BHm) were supplied by sponsor (Bioport Korea. Inc, Busan, Korea) as light to deep reddish powders as follows. All test materials were stored in a 4℃ refrigerator to protect from light and moisture, and they were well dissolved upto 40 mg/ml in distilled water, at least. Some specimens of BHj (Code BHjBPK2016Ku01), BHa (BHaBPK2016Ku01) and BHm (BHmBPK2016Ku01) were deposited in the herbarium of the Medical Research center for Globalization of Herbal Formulation, Daegu Haany University, respectively. All test materials - BHj, BHa and BHm were orally administered in a volume of 10 ml/kg, once a day for 84 days from 4 weeks after OVX operation, and 17β-estradiol was subcutaneously treated on the dorsal back skins in a volume of 0.2 ml/head, also once a day for 84 days from 4 weeks after OVX. BHj 400 mg was dissolved in distilled water 10 ml and administered in a volume of 10 ml/kg (equivalence to 400 mg/kg). BHa and BHm 400, 200 and 100 mg were also dissolved in distilled water 10 ml and administered in a volume of 10 ml/kg as equivalence to 100, 200 and 400 mg/kg, respectively. In addition, 17β-estradiol (Sigma-Aldrich, St. Louise, MO, USA) 0.03 μg was dissolved in 0.2 ml of sterilized saline, and subcutaneously treated on the dorsal back skins in a volume of 0.2 ml/mouse (as equivalence to 0.03 μg/head/day) according to the previous established methods [24-26].
Menopause Induction Via Bilateral OVX
Mice were anesthetized with 2 to 3% isoflurane (Hana Pharm. Co., Hwasung, Korea) in the mixture of 70% N2O and 28.5% O2 and were maintained with 1 to 1.5% isoflurane in the mixture of 70% N2O and 28.5% O2. The surgical protocol was carried out according to established methods [6,9] as follows. The OVX treatment group underwent open surgery involving bilateral OVX via a midline incision of linea alba. Following surgery, the incision was closed in two layers. The muscular layers were sutured independently from peripheral tissues using dissolvable 3-0 vicryl sutures, and the skin closed by continuous sutures using silk (3-0). The second group of mice (n=8) underwent a sham operation, in which a similar incision in the linea alba was made, but bilateral OVX were not performed.
Body Weight Measurements
Changes of body weight were measured once a week, at least from at OVX, 1 day before administration, initiation of administration to sacrifice using an automatic electronic balance (Precisa Instrument, Dietikon, Switzland), respectively. At OVX, initiation of administration and at a termination, all experimental animals were overnight fasted (water was not; about 18 h) to reduce the differences from feeding. In addition, body weight gains were also calculated as follow: OVX recovery/induced periods (28 days) = [Body weight at initial test substance treatment – body weight at the day of OVX surgery]; and after administration (84 days) = [Body weight at sacrifice – body weight at initial test substance treatment].
Food Consumption Measurements
All mice were allocated in individual cages contained 150 g of diets, and reminder amounts of supplied diets were measured at 24 h after feed supply using an automatic electronic balance (Precisa Instrument, Dietikon, Switzland). These are regarded as individual daily food consumption of mice (g/24 h/mouse). These measurements were conducted six times during administration, at 1, 3, 5, 7, 28, 56 and 83 days after first administration, respectively.
Serum Estradiol
For serum biochemical analysis, ~1 ml whole blood was collected from the vena cava at sacrifice and was separated from the serum by centrifugation at 21,000 x g for 10 min at 4℃ using a clotting activated serum tube. All serum samples were frozen at ‑150℃ until they were assayed. Serum estradiol contents were measured using the chemiluminescent immunoassay technique (ECLIA, Roche e411 immunoassay analyzer, Roche, Penzberg, Germany) from the separated serum at sacrifice in all individual mice, respectively.
Uterus Weight Measurements
At sacrifice, the uterus including vagina located in abdominal cavity were collected after eliminations of the surrounding connective tissues, and then the weights were measured at g levels regarding absolute wet weights. To reduce the individual body weight differences, the relative weights (% of body weight) were also calculated using body weight at sacrifice and absolute wet weight as follow: Relative uterus weight (% of body weight) = [(absolute uterus weighs/body weight at sacrifice) x 100].
Uterus Histological Procedures
Sampled tissues were fixed in 10% neutral buffered formalin (NBF). After paraffin embedding, 3-4 μm serial sections were prepared. Representative sections were stained with hematoxylin and eosin (H&E) for light microscopically examination. After that the histological profiles of uterus were observed under light microscope (Model Eclipse 80i, Nikon, Tokyo, Japan). In addition, total full, mucosa and epithelial thicknesses of the uterus (μm/ uterus) were also detected with percentages of uterine glands located in the mucosa (%/mucosa of uterus) using an automated image analyzer, respectively.
Statistical Analyses
All values for the eight mice in this experiment were expressed as means ± standard deviation. Multiple comparison tests for the different dose groups were conducted. Variance homogeneity was examined using the Levene test. If the Levene test indicated no significant deviations from variance homogeneity, the data were analyzed using the one-way analysis of variance test followed by the least‑significant differences test to determine which group comparisons were significantly different. When significant deviations from variance homogeneity were observed on the Levene test, the non-parametric Kruskal-Wallis test was conducted. When a significant difference was observed on the Kruskal-Wallis test, the Mann-Whitney U test was conducted to determine the specific pairs of groups that were significantly different. Statistical analyses were conducted using the SPSS for Windows software package (ver. 14.0; SPSS Inc., Chicago, IL, USA).
Results
Effects on Body Weights and Gains in Three Different Types of BH Preparations-Treated Mice
We selected eight mice in group showing more increases of body weights as compared with sham-operated mice, and regarded as good OVX animals at 27 days after OVX surgery (32.221.09 g of OVX mice, ranged in 30.10~34.00 g; 28.980.95 g of sham-operated mice, ranged in 27.60~30.20 g, respectively), consequently, significant (p<0.01) increases of body weights were detected in OVX control as compared to those of sham control mice with significant (p<0.01) increases of body weight gains during 4 weeks of OVX recovery/ induce and 84 days administration periods, in this experiment. However, significant (p<0.01 or p<0.05) decreases of body weights were demonstrated in estradiol, estradiol, BHj 400 mg/kg, BHa and BHm 400, 200 or 100 mg/kg treated mice from 28, 42, 49 and 56 days after initial administration as compared with OVX control mice, respectively. In addition, all 8 types of test substance treated mice showed significant (p<0.01) decreases of body weight gains during 84 days of treatment periods as compared with OVX control. Especially, BHj administered OVX mice showed similar favorable inhibitory activities on the OVX-induced body weight increases as compared with those of equal dose of BHa, and BHm treated OVX mice showed similar or slightly more favorable inhibitory effects on body weight increases as compared with equal dose of BHa administered OVX mice, respectively (Table 1).
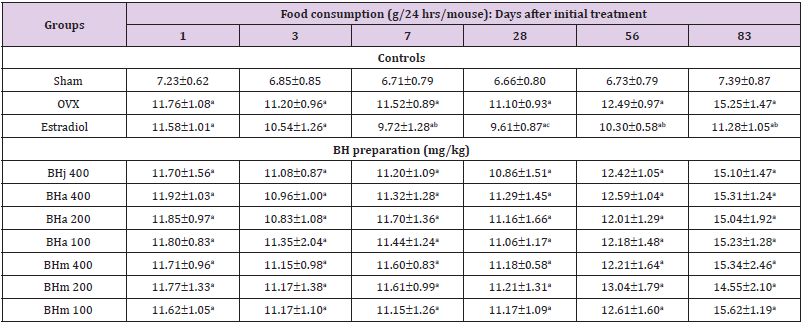
Table 1: Food consumptions in sham operated or OVX ddY mice.
Note: Values are expressed mean ± standard deviation (n=8). Three different dosages of BH preparation were orally administered, and 17β-estradiol was subcu- taneously injected at a dose of 0.03 μg/head on the dorsal back skins, once a day for 84 days from 28 days after OVX surgery. aP<0.01 vs. sham control; bP<0.01 and cP<0.05 vs. OVX control, determined by least significant difference test.
Effects of Three Different Types of BH Preparations on the Food Consumption
OVX control mice showed significant (p<0.01) increases of food consumption as compared with sham control mice at all six measured times, 1, 3, 7, 28, 56 and 83 days after initial administration, respectively. Anyway, no meaningful or significant changes on the daily food consumption were observed in all BHj 400 mg/kg, BHa and BHm 400, 200 or 100 mg/kg treated mice as compared with OVX control mice at all six measuring points in this study. However, estradiol subcutaneously treated mice showed significant (p<0.01 or p<0.05) decreases of food consumption from 7 days after initial administration as compared with OVX control mice to 83 days after initial administration, respectively (Table 1).
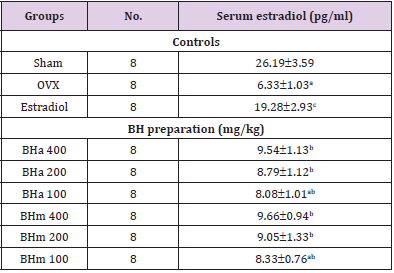
Effects of Serum Estradiol in three Different Types of BH Preparations-Treated Mice
Estradiol: Significant (p<0.01) decreases of the serum estradiol levels were deteted in OVX control mice as compared with sham control mice. However, significant (p<0.01) increases of the serum estradiol levels were demonstrated in all test substance treated mice including BHm 400 mg/kg treated mice as compared with OVX control mice, respectively. Especially, BHj administered OVX mice showed similar favorable inhibitory activities on the OVXinduced serum estradiol level decreases as compared with those of equal dose of BHa, and BHm treated OVX mice showed similar or slightly more favorable inhibitory effects on the serum estradiol level changes as compared with equal dose of BHa administered OVX mice (Table 2 and Figure 1). Our results indicated that three different types of BH preparations causes estrogenic activities.
Table 2: Serum estradiol levels in controls or BH treatment groups.
Note: Values are expressed mean ± standard deviation (n=8). Three different dosages of BH preparation were orally administered, and 17β-estradiol was subcu- taneously injected at a dose of 0.03 μg/head on the dorsal back skins, once a day for 84 days from 28 days after OVX surgery. aP<0.01 vs. sham control; bP<0.01 and cP<0.05 vs. OVX control, determined by least significant difference test.
Figure 1: Serum estradiol levels in sham operated or OVX ddY mice. Values are expressed mean ± standard deviation (n=8). ap<0.01 as compared with sham control; bp<0.01 as compared with OVX control, determined by Mann-Whitney U test.
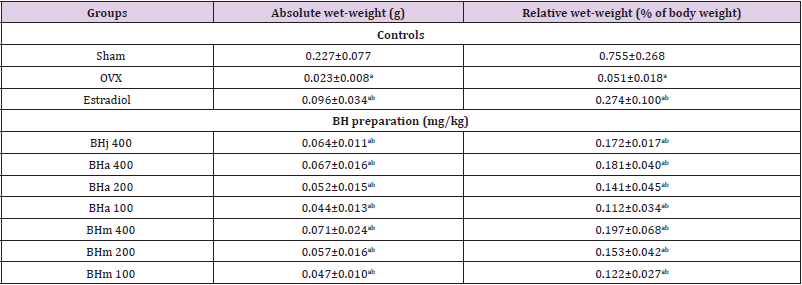
Effects of Uterus Weights in Three Different Types of BH Preparations-Treated Mice
Significant (p<0.01) decreases of the uterus absolute and relative wet weights were observed in OVX control mice as compared with sham control mice, respectively. However, significant (p<0.01) increases of the uterus weights were noticed in all test substance treated mice including BHj 400 mg/kg as compared with OVX control mice, respectively. Especially, BHj administered OVX mice showed similar favorable inhibitory activities on the OVX-induced uterus absolute and relative weight decreases as compared with those of equal dose of BHa, and BHm treated OVX mice showed similar or slightly more favorable inhibitory effects on the uterine weight decreases as compared with equal dose of BHa administered OVX mice (Table 3). Our results indicated that three different types of BH preparations causes estrogenic activities.
Table 3: Uerus weights in sham-operated or OVX ddY mice.
Note: Values are expressed mean ± standard deviation (n=8). Three different dosages of BH preparation were orally administered, and 17β-estradiol was subcutaneously injected at a dose of 0.03 μg/head on the dorsal back skins, once a day for 84 days from 28 days after OVX surgery. ap<0.01 as compared with sham control by MW test; bp<0.01 as compared with OVX control by MW test.
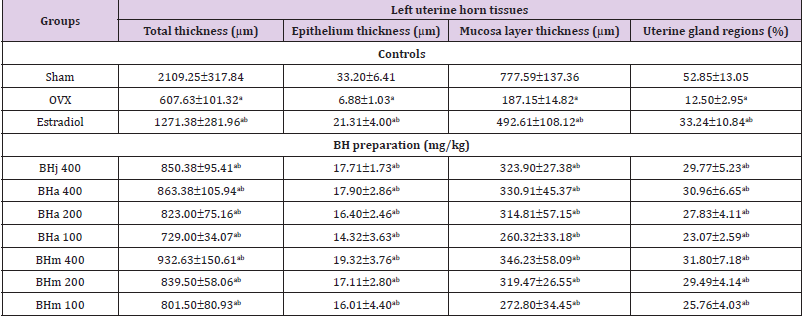
Changes in Uterus Histopathology
Significant (p<0.01) decreases of the total, mucosa, and epithelial thicknesses of the uterus, and of the percentages of uterine glands in the mucosa were demonstrated in OVX control mice, due to estrogen-depletion related disused atrophic changes, respectively. However, significant (p<0.01) increases of the total, mucosa, and epithelial thicknesses of the uterus, and of the percentages of uterine glands in the mucosa were detected in all test material treated mice including BHa 200 mg/kg treated mice as compared with OVX control mice, respectively. Especially, BHj administered OVX mice showed similar favorable inhibitory activities on the OVX-induced uterus total, mucosa and epithelial thickness, the percentages of uterine glands decrease as compared with those of equal dose of BHa, and BHm treated OVX mice showed similar or slightly more favorable inhibitory effects on the uterine atrophic histopathological changes as compared with equal dose of BHa administered OVX mice (Table 4 and Figure 2).
Table 4: Histopathology-histomorphometry for the uterus in sham-operated or OVX ddY mice.
Note: Values are expressed mean ± standard deviation (n=8). Three different dosages of BH preparation were orally administered, and 17β-estradiol was subcutaneously injected at a dose of 0.03 μg/head on the dorsal back skins, once a day for 84 days from 28 days after OVX surgery. ap<0.01 as compared with sham control by MW test; bp<0.01 as compared with OVX control by MW test.
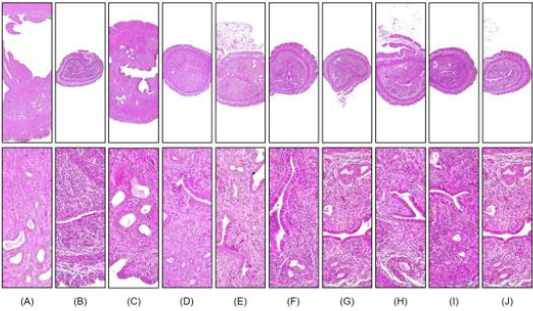
Figure 2: Representative histological images of the left uterus horn, captured from sham operated or OVX ddY mice after hematoxylin and eosin staining.
A. Sham-operated and distilled water-treated sham vehicle control mice;
B. distilled water treated OVX control mice;
C. 17β-estradiol (0.03 μg/head)-treated OVX mice;
D. 400 ml/kg BHj-treated OVX mice;
E. 400 ml/kg BHa-treated OVX mice;
F. 200 ml/kg BHa-treated OVX mice;
G. 100 ml/kg BHa-treated OVX mice;
H. 400 ml/kg BHm-treated OVX mice;
I. 200 ml/kg BHa-treated OVX mice; and
J. 100 ml/kg BHa-treated OVX mice. Scale bar, 120 μm.
Discussion
The berries are a rich source of ascorbic acid and phenolic components, particularly anthocyanins, flavonoids and low molecular weight phenolic acids [19,20]. These compounds have been reported to have multiple biological activities including strong antioxidant activity [20]. Especially BH extracts have been showed the strongest antioxidant potent among 12 types of colored berries [21], and phenolic rich extract of BH has been shown to possess anti-inflammatory and wound-healing effects in vitro and in vivo [22] and skin protective effects to ultraviolet-induced damages [23] with less toxicity [27]. The present study demonstrated that dried pomegranate concentrate powder enhanced the anti-climacteric effects of red clover in OVX rats. Therefore, we suggest that three different types of BH preparations is an attractive ingredient with anti-OVX benefits. OVX mice exhibited a significant decrease in uterine weights along with marked decreases in serum estradiol levels and associated uterine atrophic changes, including decreases in total, mucosa and epithelial thicknesses, and uterine glands in the mucosa. However, these estrogen‑deficient uterine atrophies were significantly inhibited by three different types of BH preparations treatment. As estrogens are shown to act on numerous female target organs, such as the uterus, vagina, and skeletal and cardiovascular systems [28], menopausal women may experience climacterium symptoms due to lack of estrogen [29,30]. Loss of estrogen is accompanied by atrophy of the uterus and vagina [30,31]. Phyto estrogenic effects of is flavonoids cause increases in uterine masses via uterine water inhibitions and/or a cell proliferation [28,32], which are mediated through ERα [16-18].
Conclusion
The results obtained in this study suggesting all three different types of BH preparations (BHj, BHa and BHm) showed favorable effects on serum estradiol and uterus weight, in the current experiment. It, therefore, is expected that BH preparations will be promising as a new potent refinement agent for relieving the climacterium symptoms.
| For more Articles on : https://biomedres01.blogspot.com/ |




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.