Cobalt Doped TiO2/rGO Nanocomposites as Highly Efficient Photocatalyst for Water Purification
Introduction
Photocatalysis is a crucial research filed, which solves the
problem of energy and environmental pollution in the world in
an economical and sustainable way [1]. Titanium dioxide (TiO2),
as the most common candidate among various semiconductor
photocatalysts, has been widely utilized in the environmental filed
because of its high activity, long-term stability and low toxicity [2-6].
However, because of its wide band gap (Eg≈3.2 eV for anatase type
TiO2) and high recombination rate of electron-hole pairs, TiO2 can
solely adsorb the UV light which is merely 3~5% of solar spectrum,
resulting in low utilization of the majority of the solar energy [7-
9]. In order to overcome these drawbacks, various improvement methods have been explored including heterogenous composition
[10,11], element doping [12,13], surface modification and the
like. Among them, the element doping of TiO2 photocatalysts has
been considered as a feasible method to improve the interfacial
charge-transfer efficiency, narrow the band gap and delay the
recombination of carriers. Up to now, transition metal such as
Co [14,15], Pt [16], Sn [17] and Fe [18] has been reported to be
successfully doped into TiO2, and the light response wavelength
of the obtained materials showed significant red-shift. According
to literatures, transition metals cobalt is considered as one of the
best candidates to reduce the electron-hole recombination rate
and transfer the adsorption edge to the visible light region [19-
21]. The cobalt oxide-loaded TiO2 (TiO2-CoO) support with reduced
graphene oxide (rGO) was fabricated by sol-gel method and utilized
to remove 2-chlorophenol (2-CP).
The removal efficiency of 2-CP was 98.2% with the ternary
nanocomposite in the visible region [22]. The ternary rGO-TiO2/Co3O4
nanocomposites were successfully prepared by co-precipitation
method, and exhibited the highest degradation performance of
methylene blue (MB) and crystal violet (CV) dye under visible light
[23]. As a result, cobalt doped TiO2 photocatalysts have shown
superior performance in degrading various organic pollutants.
Graphene oxide (GO), due to its excellent electrical conductivity,
large surface area and chemical stability, has attracted wide
attention as a substrate for promoting the uniform distribution of
heterojunction materials and enhancing the photocatalytic activity
[24-28]. Due to the conjugated structure of GO, the nanocomposite
of modified TiO2 supported with graphene oxide were the perfect
combination to enhance the charge separation during the electrontransfer
processes. Therefore, the coupling of graphene oxide
with some semiconductors has received particular attention in
recent years [6]. In this paper, Cobalt doped TiO2/rGO composites
was successfully fabricated through hydrothermal method for
MB degradation. The results demonstrated that the Co-TiO2/rGO
composites remarkably enhanced the MB degradation efficiency.
Furthermore, recycling degradation experiments revealed
excellent stability of the fabricated Co-TiO2/rGO nanocomposites
for treatment of target contaminant.
Materials and Methods
Materials
Cobalt nitrate hexahydrate (Co(NO3)2·6H2O, 98%), Tetrabutyl titanate (C16H36O4Ti), glacial acetic acid (C2H4O2), ethanol (C2H5OH) and macrogol 400 (HO (CH2CH2O) n H) were obtained from He Dong Hong Yan reagent factory of Tian Jin. Natural flake graphite (≥99.85%) was purchased from Sinopharm Chemical Reagent Co. Ltd.
Catalysts Synthesis
Co-TiO2 catalysts were prepared by one-step hydrothermal method. In a typical synthesis procedure, 10mL tetrabutly titanate was dissolved in ethanol (20mL) to form homogenous solution “A”, whereas 0.02g Co (NO3)2·6H2O dissolved in a solution of ethyl alcohol, glacial acetic acid, macrogol 400 and deionized water to form solution “B”. Subsequently, solution “A” was introduced into solution “B”, and the obtained dispersion was heated at 180 °C for 5h. In following step, the prepared catalysts were washed by centrifugation with ethanol and dried at 80 °C. The obtained composites were light yellow particle and calcined in a muffle furnace at 500 °C for 3h, and the obtained sample was named as Co- TiO2. The GO was synthesized by modified Hummers method. 20mg GO powder was dispersed in a solution of deionized water (40mL) and ethanol (20mL) through 30min ultrasonic, and then 200mg Co-TiO2 was introduced to the GO suspension under vigorous stirring. Subsequently, the solution was heated at 140 °C for 5h. The resulting precipitate was washed with deionized water and dried at 80 °C. The final composites powders were labelled as Co-TiO2/rGO- 2. For comparison, the samples prepared by adding 10mg, 30mg GO powder were denoted as Co-TiO2/rGO-1 and Co-TiO2/rGO-3, respectively.
Characterization Methods
The purity and crystallinity of the prepared samples were collected by Bruker D8 Advance X-ray diffraction (Germany) with Cu Kα radiation. The morphology of the photocatalysts was characterized via Scanning electron microscope (Hitachi SU-4800). The Ultraviolet-Visible (UV-Vis) diffuse reflectance spectra (DRS) were implemented by using UV-3600 Plus. X-ray photoelectron spectroscopy (XPS) were obtained by ESCALAB 250XI (ThermoFischer Electron Corporation, USA). Electrochemical measurements were carried out on CHI 660E electrochemical workstation.
Photocatalytic Degradation
The photocatalytic efficiency of Co-TiO2/rGO samples was investigated with MB degradation under visible light. The visible light source was Perfect 300W Xe-lamp (with a 420nm cut-off filter). In each experiment, 20mg of Co-TiO2/rGO composite were added to 150mL MB solution (20mg/L). The suspension was stirred in the dark for 60min to ensure the attainment of adsorption-desorption equilibrium. 5mL sample solution was extracted at predetermined time and analyzed by UV-3600 plus. The removal efficiency (R) of MB was calculated by Eq. (1).

It was expected that the degradation of the MB obeyed the pseudo-first-order reaction kinetics as follows:

where C0 (mg/L) was the initial concentration of MB, Ct (mg/L) was the concentration of MB at time t, k was the kinetic constant.
Results and Discussion
Structure Characterization
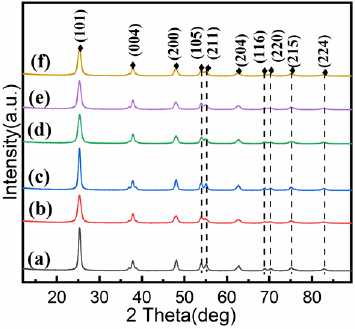
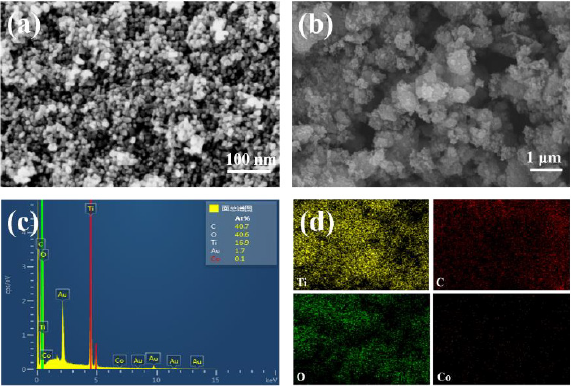
Figure 1 were the XRD patterns of the as-prepared nanocomposites. It was clear that all samples exhibit similar diffraction peaks. The peaks located at 2θ = 25.34°, 37.85°, 47.99°, 54.04°, 62.67°, 68.79°, 70.31°, 75.05° and 82.49°, which could be indexed to (1 0 1), (0 0 4), (2 0 0), (1 0 5), (2 0 4), (1 1 6), (2 2 0), (2 1 5) and (2 2 4) planes of anatase TiO2, demonstrating the high purity and good crystallinity of the samples [29]. The diffraction peaks of Co were not observed, which might be owing to the low content of Co (NO3)2· 6H2O or the cobalt ions were uniformly dispersed into the anatase crystallites. It was noteworthy that the peak intensity corresponding to the (2 1 1) crystal plane in the cobalt-doped nanocomposites varied, indicating that the presence of Co2+ ions around Ti4+ [30]. No significant diffraction peaks were noticed for XRD patterns of Co-TiO2/rGO nanocomposites when compared with Co-TiO2 nanoparticles, which was described the low rGO content in the composite, or of the TiO2 loading on the rGO surface [31,32]. Surface morphology of the as-prepared composites was assayed through SEM analyses. It could be seen from Figure 2a that the Co- TiO2 particles were subsphaeroidal and well-dispersed. Figure 2b showed that the agglomeration occurred when subsphaeroidal Co- TiO2 particles were combined with graphene sheets. The element composition of the Co-TiO2/rGO-2 nanocomposites were confirmed by EDS analysis. In the element mapping images (Figures 2c & 2d), C, Ti, O and Co disperse uniformly in the selected area of Co-TiO2/ rGO-2, suggesting that cobalt atoms were successfully doped into the composites. According to these images, the cobalt atoms were evenly distributed in TiO2 particles, indicating that the interaction between cobalt and TiO2 particles was excellent in the hydrothermal synthesis procedure [15].
Figure 1: XRD patterns of
a) TiO2;
b) Co-TiO2;
c) TiO2/rGO;
d) Co-TiO2/rGO-1;
e) Co-TiO2/rGO-2;
f) Co-TiO2/rGO-3
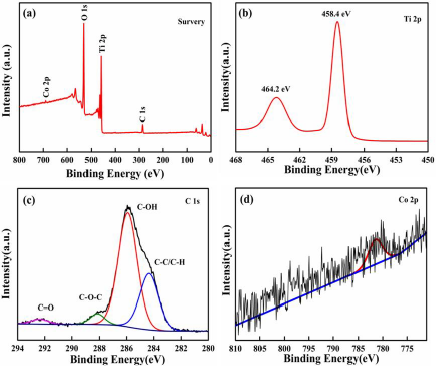
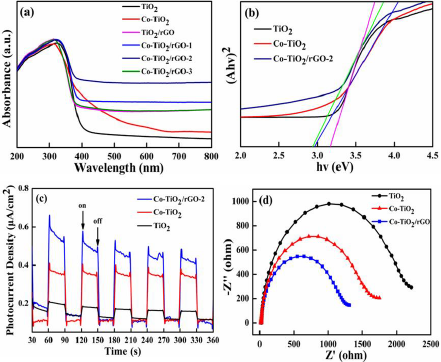
The chemical oxidation state of Co-TiO2/rGO-2 nanocomposites were measured by XPS analysis (Figure 3). As shown in Figure 3a, the XPS survey spectrum of the Co-TiO2/rGO-2 presented that C, O, Ti and Co elements could be revealed, which could consistent well with the result of EDS element mapping. The spectrum of Ti 2p (Figure 3b) exhibited two main peaks at 464.2 and 458.4eV, which were assigned to the Ti 2p1/2 and Ti 2p3/2 [33]. The C 1s spectrum of Co-TiO2/rGO-2 composite was fitted into four peaks at 292.3eV, 288.1eV, 285.9eV and 284.3eV, which were signed to C= O, C= O= C, C= OH and C= C/C= H, respectively [34,35]. In the Co 2p core level of the Co-TiO2/rGO-2 nanocomposites (Figure 3d), the peak appearing at 781.2 eV corresponded to Co (II) ions [36,37]. The optical property of TiO2 and Co-TiO2/rGO was inspected by UV-Vis adsorption spectra, as displayed in Figures 4a & 4b. Pure TiO2, with equal to 3.18eV and adsorption edge at 390nm, showed almost no visible light adsorption. Compared with the adsorption edge of pure TiO2, a strong light adsorption intensity at approximately 430nm was observed for the Co-TiO2/rGO-2 composites, which was associated to the formation of Ti-O-C bonds, resulting in reduced excited photons energies and hence low band gap energy [38]. As a result, the visible light adsorption efficiency of Co-TiO2/rGO-2 can be effectively enhanced due to the cobalt cations and rGO, which is beneficial to improving the photocatalytic degradation activity.
In order to reveal the behaviors of charge transfer and separation in the prepared photocatalysts, the photocurrent response and electrochemical impedance spectroscopy (EIS) were recorded [39]. Figure 4c showed the transient photocurrent responses of TiO2, Co-TiO2 and Co-TiO2/rGO-2 composites. It could be found that the photocurrent densities of Co-TiO2/rGO- 2 composites were significantly higher than that those of other samples, implying the efficient separation efficiency of electronhole pairs. Figure 4d exhibited EIS changes of TiO2, Co-TiO2 and Co- TiO2/rGO-2 composites. It was clearly observed that the Co-TiO2/ rGO-2 possessed much smaller arc radius relative to TiO2 and Co- TiO2, indicating that Co-TiO2/rGO-2 had lower resistance and faster separation of electron-hole in the charge transfer processes, which could well correspond to the photocurrent results.
Figure 4:
a) UV-Vis diffuse reflectance spectra of TiO2, Co-TiO2, TiO2/rGO and Co-TiO2/rGO;
b) Plot of Kubelka-Munk function versus band gap energy of TiO2, Co-TiO2 and Co-TiO2/rGO-2;
c) The transient photocurrent density of TiO2, Co-TiO2 and Co-TiO2/rGO-2;
d) Electrochemical impedance spectra of Nyquist plots of TiO2, Co-TiO2 and Co-TiO2/rGO-2.
Photocatalytic Performances
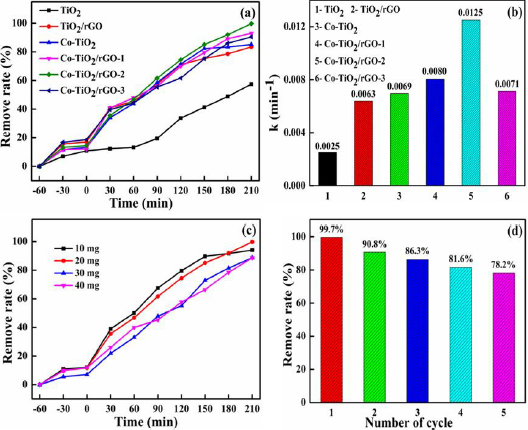
The photocatalytic performances of the TiO2, Co-TiO2, TiO2/rGO and Co-TiO2/rGO composites were evaluated by degradation MB. As exhibited in Figure 5a, the Co-TiO2/rGO-2 nanocomposites had the highest photocatalytic performance. For pure TiO2 nanoparticles, only 57.4 % of the MB was removed following 210 min under visible light irradiation. Nonetheless, the removal percentage of MB by TiO2/rGO and Co-TiO2/rGO-2 nanocomposites was 83.5% and 99.7%, respectively. The enhanced activity of the Co-TiO2/rGO-2 nanocomposites might have been attributed to the introduction of Co ions and rGO. Figure 5b manifested the kinetic constant (k) of the as-prepared photocatalytic. The k value of pure TiO2 and TiO2/ rGO were 0.0025 and 0.0063 min−1, respectively. While the Co-TiO2/ rGO-2 nanocomposites exhibited the highest MB photodegradation rate (0.0125 min-1), which was almost 5 and 1.98 times faster than those of the TiO2 and TiO2/rGO, respectively. To identify the optimum dosage of the photocatalyst, a series of experiments were carried out by varying the concentration of catalyst from 10mg to 40mg in 150mL of MB (20mg/L) (Figure 5c). It was realized that the removal efficiency of MB increased from 69.3% to 99.7% with the Co-TiO2/rGO-2 nanocomposites increased from 10mg to 20mg, which was ascribed to the availability of enough active sites on the catalyst surface. Whereas the remove efficiency decreased with a further increase in the Co-TiO2/rGO-2 dosage, which was ascribed to the agglomeration of the photocatalyst. Based on the above results, the optimal dosage of Co-TiO2/rGO-2 nanocomposites for MB degradation was to be 20mg. The stability and recyclability of the photocatalyst exerts great impact on the operating cost of wastewater treatment. Therefore, the stability of the photocatalysts was evaluated for the Co-TiO2/rGO-2 nanocomposites and the results were showed in Figure 5c. The study indicated that the removal efficiency of MB was still 78.2% after five recycling runs, indicating the activity of the recovered Co-TiO2/rGO-2 was stable enough for recycling. Therefore, Co-TiO2/rGO-2 nanocomposites were expected to be promising in environmental remediation because of their excellent photocatalytic activity and stability.
Figure 5:
a) Photodegradation of MB under simulated solar irradiation over the as-prepared photocatalytic;
b) The kinetic constants of the as-prepared photocatalytic for the MB photodegradation;
c) Efficient of the dosage of the MB photodegradation by Co-TiO2/rGO-2;
d) Effect of cycling times on photocatalytic efficiency on Co-TiO2/rGO-2
Proposed Mechanism for Photocatalytic Degradation of MB
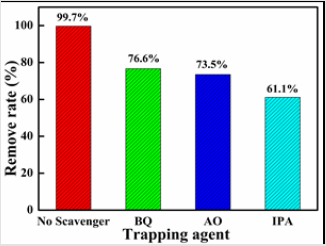
To determine the active species (such as •OH or h+ or •O2
- radicals)
and further explore the photodegradation mechanism, isopropyl
alcohol (IPA), ammonium oxalate (AO) and 1,4-benzoquinone (BQ)
were used as the radical scavengers [40,41]. The experiment data
revealed that the photocatalytic activity of Co-TiO2/rGO-2 was
decreased by adding the radical scavengers but to different degrees
(Figure 6), indicating that all the above active radical species were
responsible for the MB degradation. Notably, the photocatalytic
performance dropped sharply to 61.1% with the addition of IPA,
demonstrating that h+ radical was the main active species in the MB degradation process. Based on the above characterization and
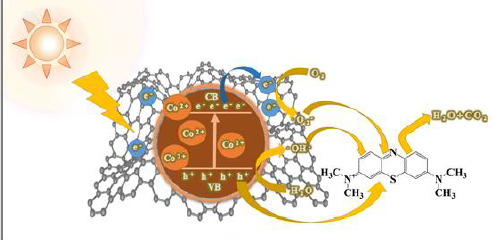
photocatalytic activity results, a plausible mechanism of Co-TiO2/
rGO-2 for MB degradation has been proposed and shown in Figure
7. The improvement of TiO2 photocatalytic performance could be
explained as follows:
1) The doping of optimal Co2+ into the lattice of TiO2
nanosheet could efficiently reduce the band gap width of TiO2 and
increase the adsorption of visible light [42,43].
2) The specific surface area of the composite increased
due to adding rGO, and more active sites could be provided for
photocatalytic activity [44].
3) Under the excitation of visible light, the electrons
generated by the conduction band of TiO2 were captured and
transferred by the graphene layer, which improved the electronholes
separation efficiency [45,46].
The electrons subsequently react with the oxygen molecules adsorbed on the surface of the catalyst to generate •O2 - to degrade MB. At the same time, the residual h+ within the TiO2 VB can be directly or through water oxidation to generate ·OH radicals, and in turn photo oxidize of MB [15]. In summary, the addition of Co metals to TiO2/reduced graphene oxide composite have demonstrated to be beneficial for degrading MB, which was consistent with the electrochemical measurements. The synergy effects of Cobalt doped TiO2 and rGO was conducive to the formation of the active sites and the facilitation of the high photocatalytic performance. Therefore, Co-TiO2/rGO composite offered an excellent combination of high activity and long-term performance durability.
Figure 7: The probable photocatalytic degradation mechanism for MB by the Co-TiO2/rGO nanocomposites.
Conclusion
In conclusion, an efficient Cobalt doped TiO2/rGO photocatalyst was successfully prepared, and the properties of Co-TiO2/rGO nanocomposites were investigated. It was noticed that the Co-TiO2/ rGO-2 revealed an excellent photocatalytic performance in the MB degradation process. Compared to TiO2, MB degradation percentage was increased from 57.4% to 99.7% in the existence of Co-TiO2/rGO- 2. This phenomenon could be explained as the special properties of reduced graphene oxide components and cobalt dopant, which facilitate the separation of photo-generated carries and extend the adsorption spectrum of TiO2 into visible region. Furthermore, the degradation percentage of MB was still obtained to 78.2% after five cycles. Therefore, Co-TiO2/rGO nanocomposites have promising applications in degradation of the organic compounds in the coloring, petroleum and leather industries.
For more Articles on : https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.