Determination of the Anti-Adhesive and Anti-Biofilm Capacity of a Wheat Extract on Staphylococcus Aureus in Farms
Introduction
Microbial contamination and infections have always been a
problem and a limiting factor for growth and animal production on
fattening farms. To combat and prevent certain livestock infections
by certain microorganisms, antimicrobial therapy has been used
in animals in recent years. Even so, several studies question this
methodology due to the increasingly frequent appearance of
resistance to these compounds. Therefore, it is necessary to find
new ways to prevent microbial infections in livestock that are
independent of substances that can generate resistance [1]. Several
studies have proposed alternative products that can be used as
additives in animal feed, with the ability to prevent adhesion and
intestinal colonization of pathogenic bacteria in animals, which
also do not generate resistance by microorganisms [2-4]. Some of
the alternative products can be such as prebiotics, probiotics, plant
extracts, organic acids, enzymes, or micro minerals. A different
problem from colonization and infection of cattle is the ability
of certain microorganisms to adhere to certain types of surfaces,
forming films of microbial communities enveloped by extracellular
matrix called “biofilms”. It is known that microbial growth in
nature is found mainly in the form of biofilms since in this way
the microorganisms present more resistance to environmental
factors and antimicrobial agents than in the free form [5]. In some
cases, low doses of antibiotics may even favour the formation and
growth of a biofilm, which indicates a natural defence mechanism
for microorganisms to avoid the toxic effect of these substances [6].
These structures are therefore much more difficult to combat
by physical or chemical methods and can cause livestock infections
more easily [7]. Some natural products have been shown to be effective
against the adhesion of certain microorganisms to surfaces,
thus presenting new study possibilities to combat the formation of
biofilms in fattening farms independently of microbial substances
[8-9]. Even so, many of these substances or natural extracts have
complex compositions and it is, therefore, difficult to determine
their activity and their ability to block the adhesion of bacterial
cells
to surfaces is in some cases purely empirical [10]. In this work, the
ability of a natural extract from rye grain to inhibit the formation and
/ or break biofilm of the pathogenic microorganism Staphylococcus
aureus (Friederich Julius Rosenbach, 1884) on different types of
surfaces of snail and poultry farms has been tested. The efficacy
of this natural extract was previously described against biofilms
formed in epithelial tissue of mammary glands in cows, causing
mastitis and subclinical infections of the mammary glands [10]. S.
aureus is a non-sporulated facultative anaerobic microorganism
that is Gram-positive cocci and is positive for catalase and coagulase
reactions. Despite being a pathogenic microorganism, it is widely
distributed in a wide variety of environments and habitats, also
forming part of the normal microbiota of the skin and mucosa of
warm-blooded animals.
It can cause a wide variety of infections and diseases, either
through infection and colonization of tissues, or through the
production of toxins, among which several types of enterotoxins
and hemolysins stand out. Among the diseases caused by this
microorganism, stand out from benign mucosal and skin infections
or gastroenteritis caused by enterotoxins to infections of internal
tissues, pneumonia, meningitis, endocarditis, or sepsis, among
others. The adhesion of S. aureus on animal tissue surfaces and the
formation of biofilms have been described as important virulence
factors [3]. It is also known that the mechanism described as
“quorum sensing” intervenes in the regulation and coordination
between the different microorganisms responsible for the creation
and maintenance of a biofilm [11-12]. In this sense, the plant extract
tested in this study has been shown to be a potential inhibitor
of biofilm formation due to its anti-adhesive properties against
Enterobacteriaceae [1,13-14].
The main objective of the work is to demonstrate the effect that
a soluble wheat extract has on S. aureus biofilm on four different
types of surfaces:
- A PVC surface, a metal surface and a cloth surface used as
a coating for rooms on snails’ farms.
- Plastic material channeling tube used to channel the
water used in poultry farm waterers.
For all surfaces, the ability of the wheat extract to prevent the
formation of S. aureus biofilm was determined, as well as the ability
to destroy an already formed biofilm and prevent its reappearance.
Material and Methods
To carry out the different tests, the 4 surfaces were divided into
strictly equal parts as follows:
- PVC, metallic and cloth material used in the snail’s farm:
different portions of 6x7cm each were made, previously disinfected
with ethanol (96%).
- Material used in the poultry farm: the channeling tube was
cut into 8 cm portions, and then sterilized at 121ºC for 20 minutes.
For all the tests carried out, an overnight culture of the
microorganism was started in rich medium BHI (Brain heart
infusion). The process was carried out at all times under sterile
conditions to avoid contamination of the samples. As a diluent for
the preparation of the different samples, a sterile solution of 50
g/L glucose and 9 g/L NaCl was used to simulate the conditions of
the poultry farm, to which 3 g/L of a supplement of amino acids
and vitamins for birds after sterilization. All tests were made at the
same concentration of wheat extract (0.29mg/100mL of solution).
Determination of the Extract’s Ability to Prevent the Formation of S. Aureus Biofilm
To determine the inhibitory capacity of biofilm formation,
100mL of each of the following sterile dilutions were prepared:
- Positive control: 50mL of the glucose saline solution to which
0.3g of the amino acid and vitamin supplement was added
with 50mL of the overnight culture of the microorganism.
- Negative control: 100mL of the glucose saline solution to which
0.3g of the amino acid and vitamin supplement with 0.29mg of
wheat extract were added.
- Sample: 50mL of the glucose saline solution to which 0.3g of
the amino acid and vitamin supplement was added with 50mL
of the overnight culture of the microorganism and with 0.29mg
of wheat extract.
Surfaces Supplied by the Snails Farm: The surface was
divided into 15 portions of 6 x 7cm, in which 50μL of the following
dilutions were inoculated:
- “Positive control” in 3 portions
- “Negative control” in 3 portions
- “Sample” in 9 portions
The 50μL of each dilution was spread homogeneously by each
portion using a sterile Digralsky loop.
A sample (see section 2.3.) was taken from the portions
identified as positive control, negative control and 3 replicates of the
“sample” solution at 0, 24 and 48h after the initial inoculation.
During the periods between the sampling, the surface was incubated
at 37ºC under aerobic conditions.
Pipes from Poultry Farm: Twelve portions of pipes (8cm/
portion) were inoculated with the following solutions:
- 3 portions with the “Positive Control” solution
- 3 portions with the “Negative Control” solution
- 6 portions with the “Sample” solution
A sample of the liquid and the surface (see section 2.3.) were
taken from the positive and negative controls and 3 replicates of
the “sample” solution at 0, 24 and 48h after the initial inoculation.
During the periods between sampling, the tubes were incubated
(with the corresponding solution inside) at 37ºC under aerobic
conditions.
Determination of the Ability of Wheat Extract to Lysate an Already Formed S. Aureus Biofilm
For the determination of the ability of the wheat extract to
lyse an already formed biofilm of S. aureus, 100mL of each of the
following sterile dilutions were prepared:
- Initial inoculum: 50mL of glucose saline to which 0.3g of the
amino acid and vitamin supplement was added with 50mL of
the microorganism overnight culture.
- Positive control: 100mL of glucose saline solution to which
0.3g of the amino acid and vitamin supplement was added.
- Negative control: 100mL of glucose saline solution to which
0.3g of the amino acid and vitamin supplement with 0.29mg of
wheat extract were added.
- Sample: 100mL of the glucose saline solution to which 0.3g of
the amino acid and vitamin supplement with 0.29mg of wheat
extract were added.
Surfaces Supplied by the Snail’s Farm: The surfaces were
divided into 10 equal portions of 6 x 7cm, in each of which 50μL of
the following dilutions were inoculated:
- “Initial inoculum” in all portions at initial time except those
corresponding to the negative control.
- “Positive control” in 2 portions on time 24h.
- “Negative control” in 2 portions at initial time.
- “Sample” in 6 portions and the two portions inoculated with
“Negative control”, on time 24h.
The 50μL of each solution was spread evenly over each portion
with a sterile Digralsky handle.
A sample (see section 2.3.) was taken from the positive control,
negative control, and 3 replicates of the “sample” solution at the
initial time and at 24h after inoculation on the biofilm previously
formed from the “initial inoculum” solution for 24h. During the
periods between inoculations and sampling, the surface was
incubated at 37ºC under aerobic conditions.
Pipes from Poultry Farm: Eight 8cm tubes were inoculated
with the following solutions:
- “Initial inoculum” in all portions at initial time except those
corresponding to the negative control.
- “Positive control” in 2 tubes on time 24h.
- “Negative control” in 2 tubes on time 24h
- “Sample” in 4 tubes on time 24h.
A sample of the liquid and the surface (see section 2.3.) Of the
positive control, negative control, and 2 replicates of the “sample”
solution were taken at the initial time and 24h, after inoculation of
the controls and samples. During the periods between inoculations
and sample collection, the tubes were incubated at 37 °C under
aerobic conditions. For the formation of the biofilm with the “initial
inoculum” solution during the first 24h, the tubes were emptied
and incubated dry, while for the following 24h they were incubated
with the corresponding solutions inside.
Sampling
On Surfaces Supplied by the Snail Farm: Sampling on these surfaces was carried out using sterile swabs moistened with 100μL of sterile Ringer’s solution in order to collect the maximum number of viables present in each portion. Subsequently, the swab was suspended in a tube with 9 mL of Ringer’s and 100μL were seeded in 3 plates of Mannitol-Salt Agar (3 replicates per time and portion). The plates were incubated at 37°C for 48h under aerobic conditions before proceeding to the colony count.
In tubes Supplied by The Poultry Farm
A. From the liquid Inside the Tubes: The liquid was
decanted into a sterile tube so as not to affect the number of viables
on the surface during handling, it was homogenized and 100μL was
used to make the corresponding dilutions with sterile Ringer. The
two most suitable dilutions were chosen to enable a representative
and reliable CFU count. From both dilutions, 100μL were seeded
in two plates of Mannitol-Salt Agar for each dilution. The plates
were incubated at 37°C for 48h under aerobic conditions before
proceeding to the colony count.
B. Of the Surfaces of The Interior of the Tubes: Subsequent
to the vacuum of the liquid inside the tubes, a sterile swab was
humidified with 100μL of sterile Ringer, and it was passed over the
entire surface of the inside of the tubes in order to take the maximum
number of viable. Subsequently, the swab was suspended in a tube
with 9mL of sterile Ringer, from which a serial dilution bank with
sterile Ringer was performed. The two most suitable dilutions were
chosen to enable a representative and reliable CFU count of both
dilutions, 100μL were seeded in two plates of Mannitol-Salt Agar
for each dilution. The plates were incubated at 37°C for 48h under
aerobic conditions before proceeding to the colony count.
Results
PVC Surface Supplied by Snails Farm
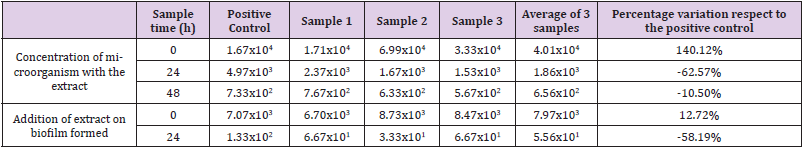
The results of the PVC surface used on the snail’s farm are shown in (Table 1). The results are expressed in CFU/unit of surface sampled. The test of the capacity of the plant extract to inhibit the formation of the biofilm on the PVC surface provided by the snail farm (Table 1), shows an increase in the number of viables of 140.12% in the samples with respect to the positive control at initial time, while, after 24 and 48h of incubation at 37ºC, they present decreases of 62.57% and 10.50% respectively with respect to the positive control. Regarding the test in which the capacity of the plant extract to destroy a biofilm on the same surface was evaluated, the counts showed an increase in the number of viable of 12.72% of the samples over the positive control at initial time, while on time 24h they showed a reduction of 58.19%.
Table 1: Counts corresponding to the PVC surface used in the snail farm. The results are expressed in CFU/total area sampled, and the percentage variation of the average of the samples inoculated with the plant extract versus the positive control.
Pipes from the Poultry Farm
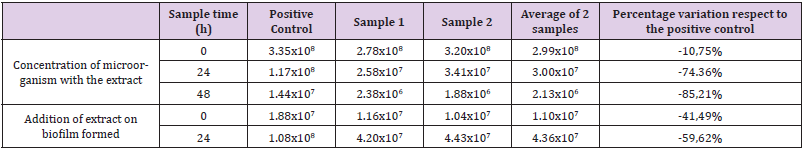
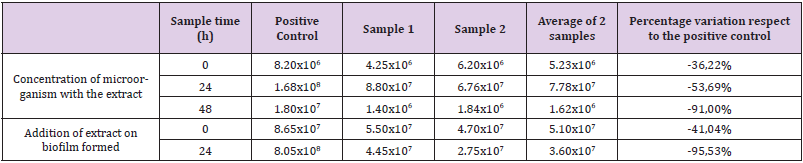
The results of the material supplied by the poultry farm for the biofilm inhibition capacity determination test are shown in (Tables 2 & 3). The results are expressed in CFU/mL of the liquid contained in each portion and in CFU/unit of sampled area. When performing the CFU counts per mL of the liquid contained within each portion, select a reduction of the percentage of viable microorganisms compared to the positive control of 10.75% at the initial moment, 74.36% at the 24h time and 85,21% at the time 48h after the inoculation of the extract When evaluating the capacity of the extract to destroy a modified biofilm, the affected counts reduced the number of viable of 41.49% of the samples on the positive control at the initial moment, while at 24 h it reduced a reduction of 59.62%. When performing the CFU counts of the interior surface of each portion, a percentage reduction of viable microorganisms was observed compared to the positive control of 36.22% at initial time, 53.69% at 24h time and 91.00% at time. 48h after inoculation of the extract. When evaluating the extract’s ability to destroy a formed biofilm, the counts showed a reduction in the number of viable of 41.04% of the samples over the positive control at initial time, while at 24h time they showed a reduction of 95.53%.
Table 2: Counts corresponding to the liquid inside the tubes used in the poultry farm. The results are expressed in CFU/mL and the percentage variation of the average of the samples inoculated with the plant extract versus the positive control.
Table 3: Counts corresponding to the interior surface of the tubes used in the poultry farm. The results are expressed in CFU/area sampled, and the percentage variation of the average of the samples inoculated with the plant extract versus the positive control.
Metallic Material Supplied by the Snail Farm
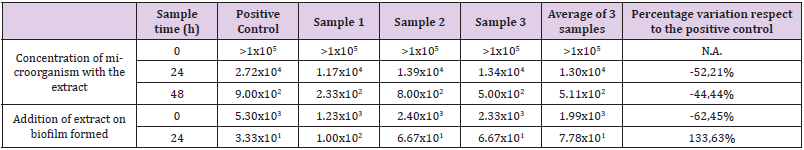
The results of the PVC surface used in the snail farm are shown in (Table 4). The results are expressed in CFU/unit of total area and percentage of variation of the samples with respect to the positive control. Viable counts on the metal surface provided by the snail farm for the test for determining the inhibitory capacity of biofilm formation, showed very high results at time 0h, which were expressed as counts greater than 105 per unit area. In these counts it was impossible to determine the percentage reduction of the samples with respect to the positive control. At 24h and 48h time, the counts showed decreases of 52.21% and 44.44% respectively. The test carried out to determine the ability of the extract to destroy biofilm formed on the same surface, showed a decrease in the number of viables of 62.45% at initial time, and an increase of 133.63% at time 24h.
Table 4: Counts corresponding to the metal surface used in the snails farm. The results are expressed in CFU/total area sampled, and the percentage variation of the average of the samples inoculated with the plant extract versus the positive control.
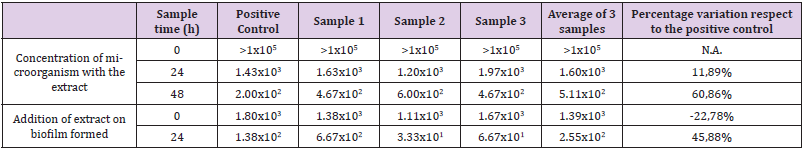
Cloth Material Supplied by the Snail Farm
The results of the fabric area used in the snail farm are shown in (Table 5). The results are expressed in CFU/unit of total area and percentage of variation of the samples with respect to the positive control. The results obtained in the test for determining the inhibitory capacity of biofilm formation, show counts above the detection limit of the technique at initial time, which were expressed as > 105 CFU/surface sampled. During this time, it was impossible to determine a percentage reduction of the samples with respect to the positive control. At 24h and 48h time, the counts showed increases of 11.89% and 60.86% respectively. The test carried out to determine the capacity of the extract to destroy the biofilm formed showed a decrease in the number of viables of 22.78% at the initial time, and an increase of 45.88%at the 24h time. Considerable microorganism counts were not obtained in any of the portions of materials tested as negative controls.
Table 5: Counts corresponding to the surface of fabric used in the snail farm. The results are expressed in CFU/total area sampled, and the percentage variation of the average of the samples inoculated with the plant extract versus the positive control.
Discussion
The way of life of some bacteria, in which the structured biofilm
structure is being formed, together with the recent increase in the
last years of resistance to antibiotics, made the treatment of diseases
caused by bacteria is very difficult [15]. On many occasions, biofilm
bacterial growth gives bacteria greater resistance to physical or
chemical agents such as antimicrobials [16-17]. Because of this,
several studies have recently been conducted to demonstrate the
potential antibiotic effect of certain molecules, including various
quorum detection inhibitors in S. aureus and other microorganisms
[15,18] or of certain probiotic strains [19]. Many of the tested
quorum-inhibiting molecules tested have been found to be
modified to prevent biofilm formation. Even so, It has been seen
that after its application on formed biofilm, the results are not as
effective [15,18]. Certain probiotic strains have also been found to
be determined to inhibit the biofilm formation of S. aureus [19]. In
this study, the potential of wheat extract to inhibit the formation of a
biofilm or destroy an already formed biofilm of S. aureus, which is
known for its biofilm-forming capacity, has been evaluated [3]. Other
studies have highlighted the possible biotechnological applications
of wheat extract [14] and its inhibitory effect on microbial films
[1]. Different surface materials (PVC surface, metallic surface, cloth
surface and drinking water channelling material) used in snail and
poultry production farms have been evaluated, in which biofilm of
S. aureus has been formed in vitro and, subsequently wheat extract
has been added to evaluate its function.
According to the results obtained in this study, a better
adherence and biofilm formation of Staphylococcus aureus can be
considered in the water channelling material supplied by the poultry
farm compared to any other surface supplied by the snail farm. In
the PVC material provided by the snail farm, the microorganism
has a moderate adherence and biofilm formation capacity, since it
starts from values of the order of 104 at the initial time for the test
for determining the inhibitory capacity of biofilm formation, and of
the order of 103 at initial time for the test of the lytic capacity on
biofilm formed. These values were reduced to orders of 102 CFU
to 101 CFU after 24 or 48 hours of incubation. (Table 1) shows the
percentage reduction in the number of viables in each test after
24 and 48 hours of incubation, suggesting an extract efficiency of
around 10% for the inhibition test and 60%for the lysis test of the
extract on said surface. Previously, high percentages of reduction
by wheat extract were also reported [1]. In the material from the
poultry farm, the results indicate a greater effectiveness of the
plant extract at high concentrations of microorganism in both tests,
presenting for the inhibition test a viable decrease of more than
85% in both liquid and surface after 48h after of inoculation.
In the test to test the ability to destroy the biofilm formed,
the percentage reduction of viables was greater than 95% after
24 hours after inoculation of the extract on the biofilm formed on
the inner surface (Table 3). Therefore, it can be indicated that the
extract has a high efficiency on this surface in both tests tested.
Both in the metallic material provided by the snail farm and in the
cloth material, the microorganism has a low adherence capacity,
since it has counts at initial time outside the detection range with
the technique used, which decrease to residual values, in the order
of 101 CFU to 102 CFU per unit area at 24 and 48h, which are also
not indicators of biofilm formation. Therefore, it was impossible to
determine the efficacy of the plant extract on these surfaces.
For more Articles on : https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.