Effects of Photodynamic Therapy on Staphylococcus Aureus Viability and Staphylocoagulase Activity, an Ex-Vivo Trial
Introduction
Staphylococcus aureus is an important pathogen with extremely high mortality in humans [1]. It has a series of harmful factors which participate in pathogenicity, such as coagulase enzyme or Staphylocoagulase (SC) [2], which distinguished S. aureus from other Staphylococcus species [3,4]. SC supports bacterial endurance inside phagocytic cells, which is the key to the pathogenic tactic of dodging host immune system reactions [5]. Accordingly, this enzyme acts to initiate the blood’s coagulation by Prothrombin Conformational Activation Mechanism (PCAM), through which N-terminal domain interacts, adheres to C-terminal of fibrinogen, and repeats the sequences [6-9]. This activity produces an active proteolytic complex (Staphylocoagulase-prothrombin complex), enhancing fibrinogen’s ability to fibrin [10]. Here comes the need for new technologies that can prevent, control, and reduce the disease process’s risk. Different strategies have been used to develop antibodies to forestall S. aureus contaminations; however, no good outcomes have been resulted [11]. It is known that some killed-vaccines and live-attenuated vaccines against S. aureus protect against varying antigens, but they need more research focus [12]. It is suggested that antimicrobial photodynamic treatment (PDT) represents a modality to treat S. aureus infection.
The PDT has a potential mechanism that can reduce Staphylocoagulase activity at a specific dose of laser irradiation along with the administration of a photosensitizer to increase the rate of cellular apoptosis [13]. This mechanism depends on Reactive Oxygen Species’ production (ROS) from cytological photochromophores of irradiated cells. The photokilling effects of MB-based PDT towards S. aureus have been described decades ago. The present study aims to evaluate a new aspect of the use of PDT on S. aureus. We have assessed the action of 650nm diode laser alone and with different concentrations of MB as an external photosensitizer to reduce SC’s activity, which may help develop an anti S. aureus treatment.
Material and Methods
Bacterial sample. Bacterial samples were isolated from infected patients and identified using analytical profile index (API) test methods. Stock cultures were maintained on Casein Hydrolysate slant agar pH 7.4 (HiMedia) at 4ºC and were sub-cultured weekly.
Isolates were divided into six groups and labeled as follows:
a) Control group: S. aureus without treatment,
b) IRA group: S. aureus with laser irradiation,
c) 50PDY group: S. aureus suspension in 50 μg/ml MB and laser irradiation,
d) 100PDY group: S. aureus suspension in 100 μg/ml MB and laser irradiation,
e) 150 PDY group: S. aureus suspension in 150 μg/ml MB and laser irradiation,
f) and 200PDY: S. aureus suspension in 200 μg/ml MB and laser irradiation.
We prepared five replicas of each sample.
Laser Irradiation
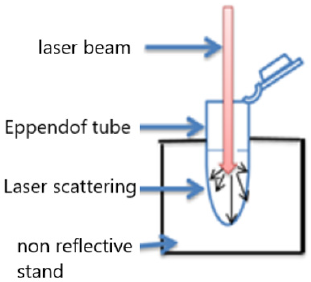
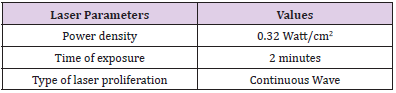
In this experiment, we used a diode laser (JD-R303, HUONJE 114 TM/ China) with 650nm wavelength and 100mW power. Table 1 shows laser parameters that we adjusted to irradiate the samples [14-16]. We used sterile Eppendorf tubes containing 1 milliliter(ml) of growth culture (5x 10⁶ cell/ml) installed in tube rack to be directly and adjacently under diode laser exposure [17]. The laser irradiation set-up was designed vertically to ensure maximum laser distribution evenly in hole suspension volume (Figure 1). Preparation of bacteria for PDT was done by taking 1 ml of the bacterial suspension after 18 hours of incubation and mixing with 1ml of MB solution. After irradiation, the samples were inoculated in 10ml of Casein Hydrolysate Broth (CHB).
Cultivation Methods
S. aureus was cultivated on 20 ml of CHB in 100 ml Erlenmeyer flasks at 35 ºC for 18 hours so that the bacterial growth culture reached the exponential phase, which is optimum for enzyme production. The cultures were shaken in a shaking incubator (LSI 3016R / Labtech Shaking Incubator) at 190 revs. min-l [17]. The bacteria were also cultured in Casein Hydrolysate Medium (CHM) (HiMedia) at PH=7.4, and then the number of colony-forming units per milliliter (CFU/ml) was obtained.
Staphylocoagulase Assay: According to Engels et al. we do the SC assay based on clotting time [18]. 20 ml of culture was centrifuged (BECKMAN COULTER/Analytical Ultracentrifuge) at 12500 g for 2 minutes. Then 0.5 ml of culture supernatant was blended with 0.5 ml of human plasma and incubated at 35 ºC for 4 hours. SC activity was calculated depending on clotting time. After the Staphylocoagulase-plasma reaction, the absorbance at 540nm was calculated for five replicates of each sample using a UV-VIS spectrophotometer (SP-3000 nano-OPTIMA, Japan). For calculating the enzyme activity (Uml-1), the following equations were applied: [19].
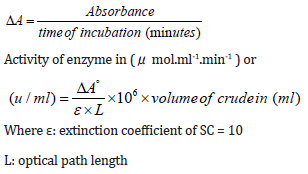
CFU Enumeration: CFU was calculated according to Thomas et al. [20]. Single Plate-Sequential Dilution Spotting (SP-SDS) technique includes preparation of CHM at pH 7.4. Each 9-cm Petridishes were divided into six sectors; each sector was labeled with the bacterial suspension’s dilution factor. A stock solution of growth culture was determined by measuring the optical density (OD) at 600 nm utilizing a 1:10 diluted stock in a UV/ VIS spectrophotometer (SP-3000 nano-OPTIMA, Japan). A serial dilution of 101–106 was set up from the 100 stocks in 1.5 ml Eppendorf tubes with 3–5 imitates and change the tips. We used sterilized distilled water autoclaved and stored for stock and dilutions preparation. Utilizing an adjusted micropipette, 20 μl of six dilutions were applied as 4–6 miniaturized scale drops in the divided sectors (sample spotting). The inoculated Petri dishes were dried off using the laminar airflow cabinet and incubated at 37˚C for 18-24 hours. The formula that we used to calculate CFU was:
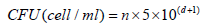
where, n = colonies number, d= dilution level yielding the countable colonies.
Statistics: We analyzed our results with SPSS software version 23.0 (IBM Inc., Armonk, NY, USA). Paired sample T-test was used to analyze the enzyme activity and CFU mean values of five replicates. We made the comparisons before and after PDT with a significance level of 0.05 and control as the dependent variable. Furthermore, we used EXCEL to present our results in a column chart associated with standard error taps.
Results
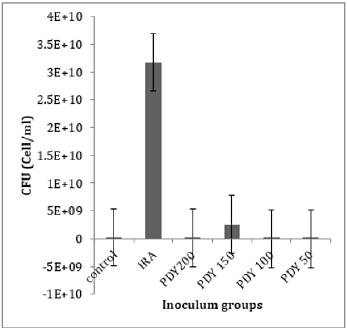
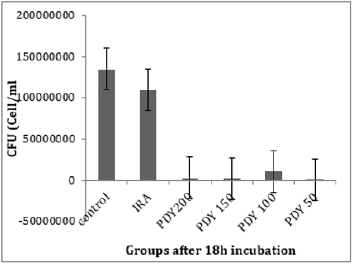
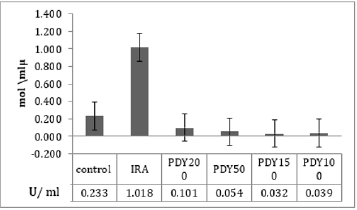
The total bacterial numbers for inoculum and exponential growth culture before and after PDT were calculated using the CFU technique described by Thomas et al. [21]. Each experiment was repeated five replicates for each sample. The mean values of inoculum in (Figure 2) show that the control, PDY200, PDY100, and PDY50 groups have the lowest values with the same mean value. In contrast, a highly increasing CFU inoculum after irradiation of the inoculum with 650nm laser for 2 minutes (IRA group) was recorded. These samples were inoculated in CHB and broth culture immediately after irradiation. It could be noticed a slight increase in total cell number means values of the PDY150 group. After 18hrs of shaking incubation, each group’s growth cultures were divided into two parts; the first part was serially diluted for a total bacterial count, then re-cultured and incubated for 18-24 hours at 37˚C-the dilution yielding acceptable colonies selected for CFU enumerations. The results show significant decay in CFU mean values of IRA groups compared to control groups. Simultaneously, the mean values of PDY groups produce the lowest bacterial count impact as a result of the highly bacterial dead ratio to inoculum volume after laser-photosensitizer treatment (Figure 3). Generally, there is a noticeable decrease in viable bacterial numbers after 18h of the incubation period. This is mainly because of the high rate of active nutrition consumption and oxygen content during shacking incubation which shortens the exponential phase compared to the stationary growth phase. The second part of the growth culture was harvested to separate and extract crude enzyme following SC activity determination steps using a spectrophotometer at 540nm. The data were analyzed by comparing means using paired sample test tables and showed a significant rising and decline in SC activity at IRA and PDY150 compared to control, respectively (Figure 4). The lowest SC activity mean values were recorded in the 150PDY group.
Discussion
The photokilling effects of MB-based PDT towards S. aureus have been described previously. The present study was designed to compare the effects of laser irradiation alone or associated with MB as a photosensitizer on S. aureus bacteria, mainly focused on Staphylocoagulase activity as a new aspect. We irradiated the groups of bacteria with a 650nm diode laser for the same exposure time to clarify the effect of laser on bacterial growth concerning the presence or absence of methylene blue as a photosensitizer, and then we calculated the SC activity in the specimens. Our findings approved that 2 minute of irradiation of S. aureus with a 650nm diode laser alone results in rising total cell numbers and SC activity. As noticed in (Figures 2 and 4), photo-biostimulation influence response to 650nm laser on inoculum (the IRA group) after 2 minutes of laser irradiation significantly increased the SC activity (P-value= 0.003). It included an increase in cell proliferation rate and biomass of bacteria after overnight culturing. These results are contradictory to the findings of Chung et al. [21]. According to Chung̓s study, where they irradiated three different bacteria species, S. aureus, E. coli, and P. aeruginosa, with nine unique laser frequencies for 15minutes, none of the laser frequencies caused noteworthy distinction in growth on any of the three organisms’ species. Our hypothesis is that exposing the micro-organisms to laser treatment for an extended period, such as Chung ̓s study, leads to thermal effect instead of photo-biostimulation, and thermal accumulation limits actively the ionization of intracellular chromophores [22,23].
Another study by Andraus et al. has reported that low-level laser therapy (LLLT) with 660nm or 808nm lasers has no bactericidal effect and no hindrance growth in the illuminated region of plates at different irradiation times (2.15 min, 1.7min and 40 seconds) [24]. We hypotheses that the form of prepared bacterial biofilm for irradiation represents a critical factor to observe the effect of LLLT on bacterial growth and its intracellular biomolecules activities. Chung and Andraus used to irradiate bacterial culture on the medium plate in an illuminated room that provides highly accumulated concentration of bacteria that prevented total absorption of laser wavelengths despite using different wavelengths, powers, intensities, and irradiation time. In the present experiment, we prepared liquid cell suspension to be irradiated and inoculated for subculturing. (Figure 3) shows a slight drop in CFU mean values of the IRA group after 24 hours of incubation compared to control despite a significant increase in the same group’s SC activity. This occurs due to accelerating cell division rate, nutrition consumption, and shifting in the lag phase after the photo-biostimulation effect. These findings match the results of Jadah et al. [25]. PDT has adequacy against a broad spectrum of Gram-positive and Gramnegative bacteria and other types of micro-organisms. It has a multi-target mechanism [26-30], autonomously affecting their protection from standard antimicrobial treatment [31-32]. This method requires a photosensitizer (PS), light, and oxygen. The PS when energized by laser light within sight of O2, produces receptive oxygen species (ROS) like superoxide (O2•-), hydrogen peroxide (H2O2), hydroxyl radical (•OH) created by type І systems, and singlet oxygen (1O2) (type Ⅱ systems) [33].
The impacts of O2•- and H2O2 are less intense than those of •OH and 1O2 since the latter two are substantially less responsive to detoxification by endogenous antioxidants. Examples of that antioxidants system are catalase, superoxide dismutase (SOD), peroxidase, regulatory genes RpoE, RpoHII, and RoHS. Conversely, no enzyme can detoxify •OH or 1O2, making them intensely cytotoxic [34]. As indicated, we can use photodynamic conventions to reduce the virulence of S. aureus without affecting total cell viability. Therefore, this trial represents the PDT protocol to battle against SC without affecting the bacteria’s total viable count. To provide an optimal cultivation condition and maximum production of the virulence enzyme (SC) from S. aureus, which occurs only in the exponential growth phase, we utilize CHB as the optimal production medium and suitable shacking incubation parameters (190 revs. Min-1, 35˚C for 18h) [22]. Photosensitizing with MB at a 150 μg/ml concentration results in significant inhibition on SC activity compared with other groups. It can be hypothesized that photodynamic influence is more effective at 150 μg/ml concentration of MB. It is the optimal concentration of MB for maximum penetration to the intracellular bacterial structure, resulting in ROS production inside and outside the plasma membrane of the bacterial cell [35]. This can be seen in (Figure 2) by sudden increases of CFU mean values in the PDY150 group. Another PDT consequence effect was distortion in RNA-related organelles that will be inherited to successive generations; therefore, after 18h of cell proliferation, significant SC activity inhibition is seen (Figure 3) in the 150PDY group. It means that photodynamic treatment can cause inherited distortion in cytoplasmic organelles, which is responsible for low production of SC, or there was a shifting in the lag phase of bacterial growth, which leads to the lowest secretion of SC. For both hypotheses, we produce attenuated bacteria with low potential of SC as a virulent factor [36]. Morphological studies by Bertoloni et al. support the first. They have concluded that irradiation of eukaryotic cells with Helium-Neon laser at 632nm increased packing of the cytoplasmic matrix and number of ribosomes until almost complete disappearance of the microorganisms [37].
In our study, laser arrangement for bacterial suspension irradiation was designed vertically to ensure maximum laser distribution evenly in hole suspension volume. The thermal effects of laser radiation on the specimens should also be discussed. Because if the number of viable bacteria in each sample decreases due to these thermal effects, it can affect the enzyme activity in the results [32]. Our results show an increase in the number of bacterial colonies after two minutes of laser irradiation alone (Figure 2). Therefore, the rise in temperature does not seem to have much effect on our results.
Conclusion
The most effective PDTs̓ parameters which reduce SC activity were diode laser at 650 nm wavelength, 100mW power, 2 minutes’ irradiation time, liquid bacterial suspension to be exposed directly to laser irradiation, and MB concentration of 150 μg/ml. These conditions cause inhibition in SC activity without affecting bacterial viability after 24 h of incubation. This method can potentially be an effective way to treat S. aureus infections, especially the methicillinresistant S. aureus (MRSA). However, serial studies associated with immune response and another virulence factor of S. aureus are still needed.
For more Articles on : https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.