Pathogenic Microorganism Detection in Invasive Pulmonary Aspergillosis(IPA) Patients Using Bronchoalveolar Lavage Fluid
Introduction
The insufficiency of detective methods during early stage of Invasive Pulmonary Aspergillosis(IPA) has lead to severe misdiagnosis [1]. Surveys and researches had been conducted to proved that the uncertainty and improper treatment could result in relatively high mortality rate [2,3]. Bronchoalveolar lavage (BAL) is the method which can collect secretory fluid in the respiratory tract and alveoli. The fluid can be separated for further analysis so that the content of the components can be quantified. It is an common method in locating the lesion site in clinical practice. Compared with other methods, for example, BALF has fewer chance of contamination rather than collecting sputum specimen. Therefore, it can improve the specificity of etiological diagnosis. At present, this technology is mainly applied in the clinical diagnosis of pulmonary bacterial infection, but there have been few reports on the evaluation of its pathogenic detection for the diagnosis of fungal infection. In this study, BALF was collected from 100 suspected patients suffering from invasive pulmonary Aspergillus infection. Three pathogenic detection methods, smear microscopy, fungal culture and PCR were used to quantify the Aspergillus examples. Then the results of these three methods would be valued under the clinical criterion. To provide assistance for the clinical diagnosis of pulmonary aspergillosis.
Method
Collection and Storage of the BALF Specimens
The ethical approval for this study was obtained by First Affiliated Hospital of Sun Yat-sen University. Before bronchoalveolar lavage fluid were collected, the patients involved in this research received detailed explanation and understood the benefits and potential danger, and had signed the consent letters for bronchoscopy procedures. The subject inclusion and exclusion criteria used in this study refer to the consensus of Chinese experts on the detections of pathogens in the bronchoalveolar lavage of pulmonary infectious diseases, which was proposed by respiratory disease branch of the Chinese Medical Association in 2017 [4]. The procedure of collecting bronchoalveolar lavage was as follows: a) 1-2mL of 2% lidocaine was injected through the biopsy hole for local anesthesia, after the bronchoscope reached the lung segment of the lavage site.
b) The top of the bronchoscope was incarcerated in the opening of the target bronchial segment or sub segment. A dose of 20-50mL sterile normal saline at 37 ℃ or room temperature was injected through the biopsy hole of the bronchoscope. After each injection, the appropriate negative pressure (less than 100cmH2O) was used to recover the liquid, and the total recovery rate was secured to be more than 40%. The intensity of negative pressure during lavage was under severe control to avoid the collapse of distal airway caused by excessive negative pressure.
c) 8-10mL of BALF specimens were collected with a sterilized plastic collector. And the specimens were placed in an incubator with ice cubes and immediately sent to -80℃ refrigerator for preservation. The examples, one hundred hospitalized patients were collected from the different hospitals (47 patients from the Guangzhou Thoracic Hospital, 44 from the Guangdong Province People’s Hospital, and 9 patients from the First Affiliated Hospital of Sun Yat-Sen University, respectively) during the period ranging from June 2018 to October 2019. These patients had been clinically suspected of having pulmonary Aspergillus infections in accordance with the standards set in EORTC/MSG criteria [5].The IPA patients were diagnosed by pathology or detecting the Aspergillus hyphae or fungal culture by tissue biopsies or BALF. All patients were checked with bronchoscopy and BALF were collected before the antifungal therapy, then the BALF were immediately transferred to a -80℃ refrigerator in the laboratory facilities. The bronchoscopy biopsies had also been performed, and pathological diagnoses had been made by the pathology departments of each hospital.
Detection of the Specimens
After being collected, The BALF examples were thawed and divided into three parts for the application of smear microscopy, culture, and PCR product analyses. Smear microscopy and culture methods were conducted in the microbiology laboratory facilities of the First Affiliated Hospital of Sun Yat-Sen University. The smear microscopy was performed using fluorescent staining techniques. In the experimental processes, 5 mL of BALF were digested with digestive fluid (dithiothreitol). Then the examples were selected and centrifuged at 3,000 r for 15 to 20 minutes. The supernatant was discarded and the sediment remained. The sediment was placed on a glass slide and one drop of fluorescent staining solution would be added. The slide was observed under microscopeby technicians. After the residual sediment was treated again with digestive fluid, it was inoculated in Sabouraud culture medium and cultured at 30℃ for one week. After the culture was completed, the colony characteristics were observed and identified according to the characteristics of colony and conidia.
BALF PCR
Primers: After the BALF had been sent for microscopy and culturing, the remaining specimens were sent to Sagene Biotech Company Ltd for PCR detection. Then, the internal transcribed spacer (ITS) primers, which serve in Aspergillus identification, were redesigned based on the methods and sequences provided by the relevant literature [6,7] (ITS-F:GCCTGTCCGAGCGTCATTG;ITSR: TTAAGTTCAGCGGGTATCCCT; size of amplified fragments: 224 bp). The primers and the experimental reaction system were verified by the pre-experimental processes. The primers were found to have good specificity, and thus could be used for the identification of Aspergillus.
DNA Extraction: A DNA extraction kit (Sagene, China) was used to extract the DNA from each specimen. Following the extraction, gel electrophoresis was performed. 3 μL of DNA was taken and added to 1Μl of loading buffer. Electrophoresis was conducted at 120 V for 25 minutes for the purpose of analyzing the quality of the extracted DNA.
Establishment of the PCR Reaction System
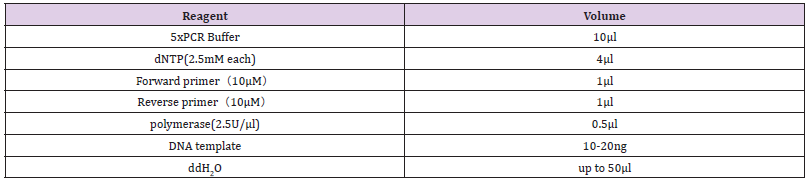
a. Reagents: The reagent types and volumes are shown in the Table 1.
b. PCR Reaction Conditions: The following were the PCR reaction conditions in this study: 98℃ for 1 minute; 98℃ for 10 seconds; 60℃ for 5 seconds; 72℃ for 5 seconds; and 72℃ for 2 minutes, for a total of 40 cycles.
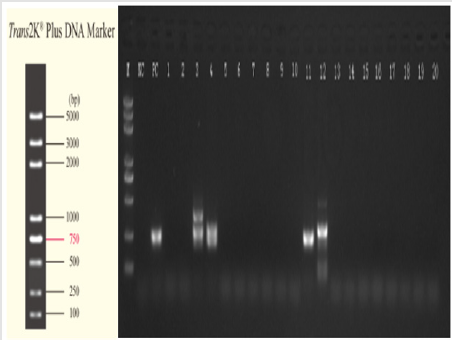
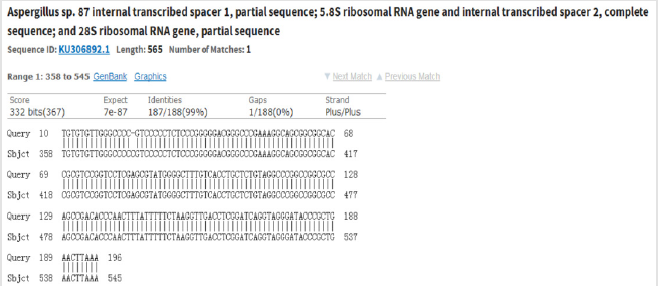
c. Preliminary Analysis: For the preliminary analysis, 3 μL PCR product was selected to perform the 1.2% agarose gel electrophoresis (Figure1) and preliminarily analyze the size of the amplified fragments, in order to determine whether or not the targeted fragments had been successfully amplified. d. Sequencing (Abi 3500) of the PCR products: Sequencing of the PCR products was conducted for the specimens which have been successfully amplified to the target fragments. Then, an NCBI BLAST comparison was performed on the sequencing results (Picture 1).
Statistical Data Method
SPSS (IBM 22.0) software was used for the statistical data in this study.
a. A Chi Square Test Method was used to compare the distributions of the three detection methods among the proven and clinical diagnosis groups. The differences were considered to be statistically significant when p<0.05.
b. A Kappa Consistency Test Method was used for the pairwise comparisons of the results of the three detection methods as a whole and among the groups, respectively.
c. The sensitivity (Sn), specificity (Sp), and positive likelihood (PRL) ratios of the three detection methods for IPA diagnoses were calculated.
Results
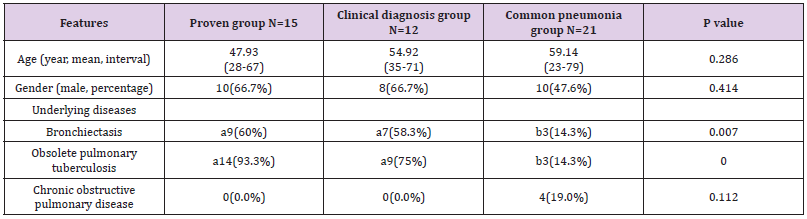
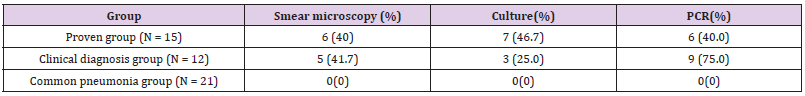
In the present study, 100 patients with suspected clinical pulmonary Aspergillus infections were registered. Through clinical examinations including pathology, radiology, etiology. Patients’ data were collected (Table 2). According to the diagnosis standards of IDSA(2016) [5], 15 patients were diagnosed by pathological data(proven group), and 12 cases were diagnosed by radiology, etiology, and other clinical examinations. The other 73 patients were diagnosed as having tuberculosis, common bacterial pneumonia, vasculitis etc. Among those patients, 21 patients with common bacterial pneumonia were used as the control group. The positive number with smear microscopy, culture, and PCR in BALF was 6 (40.0%), 7 (46.7%), and 6 (40.0%) in the proven group respectively; and 5 (41.7%), 3 (25.0%), and 9 (75.0%) in the clinical diagnosis group, respectively. In addition, the total number of patients with positive results as diagnosed using the abovementioned three methods was 0 in the common pneumonia group, as illustrated in Table 3.
χ2 = 2.187 and p = 0.335 could be obtained, there were no significant differences in the distributions of the three detection methods among the two groups (p>0.05). It was determined that the calculated χ2 and p values were 0.182 and 0.913 (proven group) and 6.241 and 0.044 (clinical diagnosis group). The statistical results showed that in the clinical diagnosis group, the BALF culture and PCR results were significantly different (p<0.05). Furthermore, in the proven group, there were no significant differences observed between the three detection methods (p>0.05).
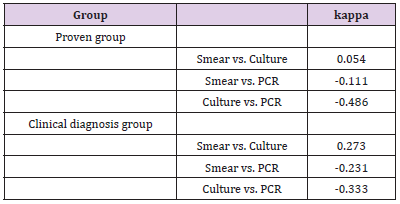
A Kappa Consistency Test Method was adopted in this study for the pairwise comparison of the results of the three detection methods as a whole, and also within the different groups. The results are shown in Table 4. As shown in the table, there was a weak consistency observed between the microscopy and culture methods. However, the results of both were found to be inconsistent with the PCR method.
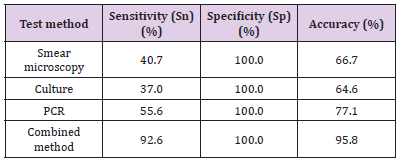
In accordance with the above-mentioned results of the Internal Kappa Consistency Test, the consistency between the smear microscopy and culture methods among the three test methods was weak. However, the results of the PCR were observed to be very inconsistent with those of the other two methods. However, it was speculated that if the microscopy, culture, and PCR methods were combined for diagnosis procedures, the diagnosis efficiency could be significantly improved. the calculation results are shown in the last row of Table 5.
Discussion
The molecular biological diagnosis method (represented by PCR) is found to be more sensitive and rapid than the traditional culture method. It could directly detect the nucleic acid molecules of the pathogens without the steps of isolation and culturing, which proved to be helpful for the early diagnoses of the disease [8]. It is known that PCR can be theoretically detected regardless of whether the pathogen are active or inactive. The positive results of the PCR were significantly greater in number than those of the culture method. However, there were no significant differences observed when compared with the smear microscopy (p> 0.05). The reason was that the IPA patients had received antifungal therapy, therefore, the positive rates of the culture method were lower than PCR. This showed fungal culture method is less the diagnosis efficiency than PCR, when antifungal therapy were conducted. However, we found that some patients were positive with BALF smear microscopy or fungal culture, but negative with PCR method. A possible cause of this is the fungal composition. The outer sides of Aspergillus (which belong to eukaryote) are wrapped by a layer of compact and hard cell wall components. The complete cell wall will inevitably reduce the extraction effects of nucleic acid substances [9].
Therefore, the interpretations of PCR results should be made in combination with other factors, such as clinical manifestations, other examination results. In our study, it was found that when smear microscopy techniques were performed for the lower sediment of BALF, the concentrations of pathogen components in those specimens were relatively high. The positive rates of smear microscopy were 40.0%, while that of common sputum was only 4.5% [10,11]. This showed the lower sediment of BALF was very high efficiency for microscopic examinations . In summary, the three examined diagnostic methods were found to have high specificity, but insufficient sensitivity. In the present study, considering the low degree of overlap in the results of the three detection methods, it was speculated that if a combination of microscopy, culture, and PCR methods was applied in diagnosis processes.
Conclusion
The pathogenic detections of bronchoalveolar lavage fluid (BALF) have been determined to be an ideal diagnostic method for Aspergillus infections. Because of the limitations of the different detection methods, The combination of BALF smear microscopy, culture, and PCR could significantly improve IPA diagnosis efficiency.
| For more Articles on : https://biomedres01.blogspot.com/ |



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.