Review Article: An Overview of Cellular Signal Transduction Pathway
Introduction
Cell signaling is a vital facet of biological life. It permits cells to handle and respond to the environment enhance development, growth and immune response etc. Cell to cell communication (signaling) is a serious part of understanding cell functions and system functions. Signal transduction is a biochemical reaction along a signaling pathway. It is the process of transferring a signal throughout an organism, especially across or through a cell [1]. Several Kinds of signaling have been identified, for example, in synapse, neurotransmitters are identified, antibody responses by antigens are started, and target cells responding to specific hormones [2].
Historical Perspectives
Signal transduction in the previous notion is traced back to 1855, at this time Scientist called Claude Bernard reported that spleen, adrenal and thyroid glands (ductless glands) released internal secretions which possess physiological effects. Also, in 1902 William Bayliss and Ernest Starling had discovered secretin [3]. After then, in 1905 Ernest Starling, was termed these secretions as hormones [4]. In 1954, Rita Levi-Montalcini was discovering the nerve growth factor and in 1962 Stanley Cohen was discover epidermal growth factor which led to more insights into the basis of the signaling of the cell, especially the growth factors [5]. In 1956, those scientists together with Earl Wilbur Sutherland’s discovery of cyclic Adenosine Monophosphate (cAMP) which motivated to redefine the endocrine, autocrine and paracrine signaling3. In 1970, Martin Rodbell was demonstrated that guanosine triphosphate disassociated glucagon from rat’s liver cell membrane receptor and activated the G-protein that highly affected the metabolism of the cell [6]. In 1972, was the time in which the term “signal transduction” was used, due to the discovery of the characterization of G Protein Coupled Receptor (GPCRs) as well as receptor Tyrosine Kinases (RTKs) [7]. Several researches used a terms like signal transmission as well as sensory transduction [8,9].
Axes of Cellular Signal Transduction
Three axes of cellular communication or signaling (Figure 1).
Environmental Stimuli
A protein at cell surface detects the chemical signals. Signal transduction relies on proteins known as receptors, signal transducer or sensor, which waits for a chemical, physical, or electrical signal [10].The nature of such stimuli can vary widely.
Ligands (Signaling Molecules): Chemical signals are called ligands or first messengers. Regardless the signal type, it must be across cell membranes and transferred throughout the body. This process is known as signal transduction. In case of the pathway of signal transduction, signaling ligands bind its receptors (receptor activation)and start the cellular response. Commonly ligands are soluble molecules from the extracellular medium such as growth factors, cytokines and neurotransmitters which bind to receptors on the cell surface [11].
Mechano Transduction (Mechanical Forces): For example, mechano-transduction forms in the central nervous system and are respond to mechano-sensation such as balance, proprioception, touch as well as hearing [12].
Osmolarity: The osmotic pressure (the osmolarity difference in between the extracellular media as well as the cytosol) is important for homeostasis. Osmotic stimuli within the cell like ionic strength, macromolecular crowd alteration, as well as alteration in the cytoskeleton or plasma membrane characters [13]. These changes are recognized by proteins called osmoreceptors or osmosensors. The best characterized osmoreceptors are that of human cellular primary cilium which transient receptor potential channels [13,14].
Thermoception (Temperature): The thermoception is primarily mediated by transient receptor potential channels. There are various thermosensory mechanisms exist in both prokaryotes and eukaryotes [ 15]. For example, the cells possess the heat-shock response, a protected mechanism which inhibits the increasing of temperatures from causing cellular damage. Increasing the temperature leads to dissociation of the inactive Heat shock factor 1 (HSF1) from its complexes within the Heat Shock Proteins (Hsp) such as Hsp90 as well as Hsp40/Hsp70. Then an expression of target genes are activated and up regulated due to the RNAmediated response to heat shock (ncRNAhsr1) and HSF1 [16].
Visual Phototransduction (Light): Generally, in the case of vision, the photoreceptor cells (light-sensitive proteins) in the eye’s retina are activated in response to light and ultimately led to the sight as well as circadian clock [17]. In the case of sight the photoreceptor in the rod and cone cells are activated in response to light detected by rhodopsin. In the circadian clock, intrinsically photosensitive retinal ganglion cells photoreceptors are activated in response to light detected by different photopigments [18].
Transduction Receptors (Sensor or Signal Transducers)
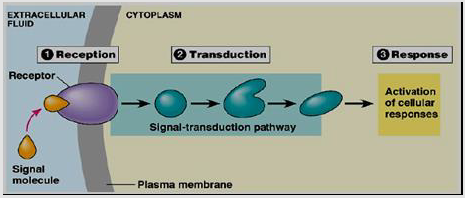
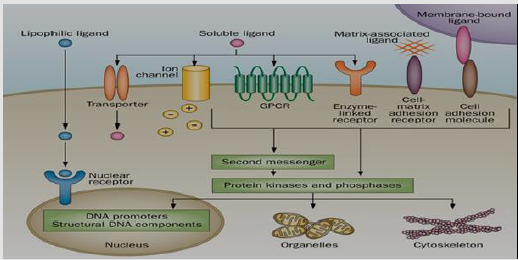
A rising of the signaling cascade occurs through many changes elicited by ligand binding (signal sensing) to its receptor [19]. Receptors binding cause activation of the primary effectors which are second messengers linked and lead to secondary effectors activation and finally cause a certain response on the cell (Figure 2) [20]. Generally, receptors are classified into two major classes: intracellular as well as extracellular receptors.
Extracellular Receptors: Extracellular receptors are integral transmembrane proteins that span the plasma membrane of the cell, with one part of the receptor on the outside of the cell and the other on the inside. The process of ligand-receptor binding is led to receptor activation through stimulating the change in the structure of the receptor inside part [21,22]. This action leads to the enzyme stimulation on domain of the receptor, leading to an enzymatic activity such as tyrosine kinase and phosphatases, and its activation, ultimately transmitting the signal through the cytoplasm [23].
Extracellular receptors are subdivided into:
a) G Protein– Associated Receptors
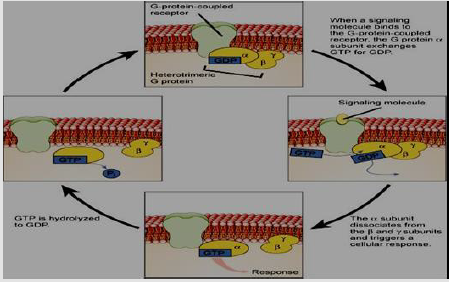
G Protein-Coupled Receptor (GPCRs) or serpentine receptors are the largest family of extracellular membrane sensors which have transmembrane domains which are bound to a heterotrimeric G protein (Gα, Gβ, and Gγ subunits which are inactive when bound to Guanosine Diphosphate (GDP). GPCRs are classified into five main subfamilies; secretin-like, rhodopsin-like, adhesion and frizzled/ smoothened as well as metabotropic glutamate. On the other hand, there are little GPCR subgroups are difficult to classify because of its low sequence similarity, such as vomeronasal receptors, Dictyostelium cyclic AMP receptors and fungal mating pheromone receptors [23]. At the moment that GPCR Senses the ligand, the receptor is conformed and changed to activate the G protein, at the same time GDP is phosphorylated to guanosinetriphosphate (GTP) by Guanine-Nucleotide Exchange Factors (GEFs), leading to bind of Gα to a GTP molecule and disconnect from the other two subunits of G-protein. This disconnection leads to exposure of the sites of subunits which can interact with other molecules [24]. Then the stimulated subunits of G protein are separated from its receptor then start signaling from various downstream effectors proteins like phospholipases as well as ion channels. Ion channels led to the emission of second messenger components. The intensity of GPCR signaling extension is detected by the ligand-receptor complex lifetimes as well as receptor-effector protein complex and the activated receptor and effectors deactivation time via the activity of intrinsic enzymatic like β-arrestin-dependent internalization or protein kinase phosphorylation [25,26] (Figure 3).
b) Enzyme-Linked Receptors
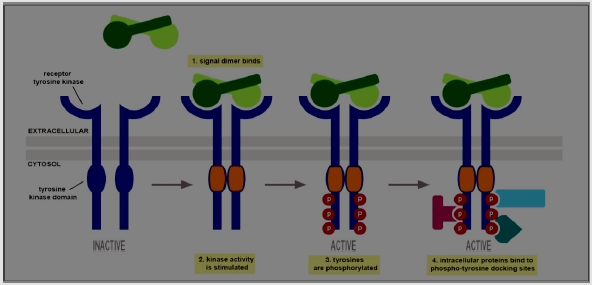
A six enzyme-linked receptors kinds have been identified like tyrosine kinases receptor; tyrosine phosphatases, Tyrosine kinase associated receptors; serine/threonine kinases; guanylylcyclases as well as histidine kinase associated receptors. Tyrosine Kinases Receptors (RTKs)are the biggest population and widely applicable. They are transmembrane proteins possess an extracellular domain that binds ligands and an intracellular kinase domain [27,28]. The main example of these receptors are growth factors receptors like Platelet-Derived Growth Factor (PDGF), Epidermal Growth Factor (EGF), Hepatocyte Growth Factor (HGF), Fibroblast Growth Factor (FGF), Nerve Growth Factor (NGF) as well as hormones. In case of signal transduction, the plasma membrane RTKs have to form dimers [29]; binding between the ligands and its receptor causes stabilization of the dimer. The interaction with the cytoplasmic domains activates tyrosine residues autophosphorylation in the RTKs domains of intracellular kinase, leading to conformational modifications. After then, the receptor’s kinase domains are stimulated, starting of cascades of downstream cytoplasmic molecules phosphorylation signaling that enhance many operations within the cell for example metabolism as well as cellular differentiation [28].
Ser/Thr and dual-specificity protein kinases signal transduction are processed through either acting downstream of RTKs, or cell-soluble or as membrane-embedded [30,31]. Like is the case with GPCRs, GTP binding proteins possessing a main function in the signal transduction from the activated cellular RTK. At that condition, the G proteins are among the Ras, Rho, and Raf families, and known as small G proteins. The small G proteins do as molecular keys that linked to the membrane via isoprenyl groups attached to their carboxyl ends. In case of activation, these proteins specify other proteins to specific membrane subdomains to be participated in the signaling. The activated RTKs led to the activation of small G proteins which are causing guanine nucleotide exchange factors like SOS1activation. The activated exchange factors stimulate excess of small G proteins, consequently magnifying the initial signal of receptor’s [32]. Histidine-specific protein kinases have a different structure than protein kinases and are present in prokaryotes, plants, as well as fungi in a mechanism of signal transduction. In this mechanism, at first a phosphate group from Adenosine Triphosphate (ATP) is transported to the kinase histidine remains, after that it transported to an aspartate remains leading to its activation on the received domain on another protein or the kinase itself [33] (Figure 4).
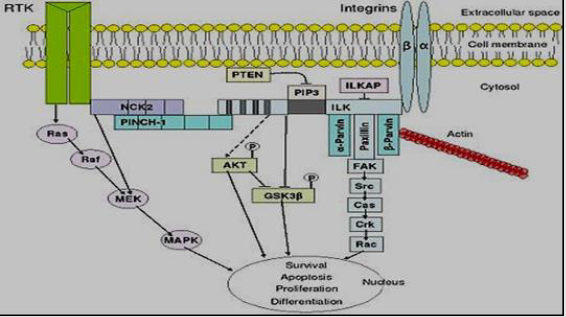
c) Cell Linkages- Integrins
Integrins, transmembrane proteins, provide important communication between the intracellular as well as the extracellular domains. They are amechanotransducers as well as signal conductors, providing a physical join between the Extracellular Matrix (ECM) and the cell’s cytoskeleton. Despite integrins have no intrinsic enzymatic activity; they would react with enzymes like kinases. They are involved in various processes within the cell, like migration, differentiation, apoptosis, ECM protein expression, growth factors activation as well as proliferation. They able to bind to ligands extracellularly and starting signal pathways intracellularly. Also, they may be activated by many cellular factors to manage the cell-environment relationship. Integrins are heterodimers, have two identified subunits (α as well as β). In humans 18 various α chains as well as 8 various β, have been identified [34,35]. The subunits react with each other and exist in either a low-affinity or a high-affinity conformation depending on the signals [36-38]. Integrins are inactively distributed on the surface of the cell. In case of its binding with proteins of ECM, they transport with the membrane of the cell to cluster and compose sites of focal adhesion (activation). These sites can after that compose their actions with many other proteins like talin, Focal Adhesion Kinase (FAK), tensin as well as paxillin. Integrin interactions between talin, vinculin, α-actinin, and paxillin supply a physical link of the ECM with the actin cytoskeleton; these linkages are vital for cellular anchorage as well as migration. Cytoplasmic β tail Phosphorylation damages the talin–integrin reaction, leading to cellular movement [39,40] (Figure 5).
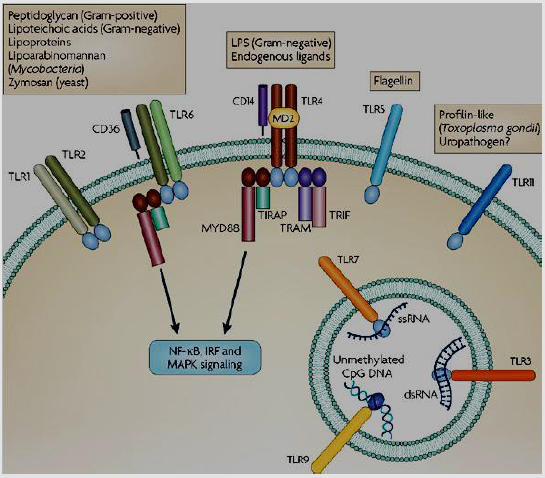
d) Immune Receptors
The most known immune receptor is the Toll-like receptors (TLRs).in case of TLRs activation; it takes adapter molecules into the cellular cytoplasm to reproduce a signal. The known signaling adaptor molecules are Myeloid differentiation primary response 88 (Myd88), TIR-domain-containing adapter-inducing interferon-β (TRIF), Toll-Interleukin 1 Receptor Domain Containing Adaptor Protein (TIRAP), as well as Translocation Associated Membrane Protein (TRAM) [41-44]. These adapters activate other intracellular molecules such as Interleukin 1 Receptor Associated Kinase 1 (IRAK1), TANK Binding Kinase 1 (TBK1),Interleukin 1 Receptor Associated Kinase 4 (IRAK4), and I-kappa-B kinase inhibitor (IKKi) which led to the signal amplification and causing genes induction or suppression depending on certain responses [42]. There are other types of Immune receptors had been identified such as T cell receptor, CD28, C-terminal Src kinase Interacting Membrane Protein (SCIMP) (Figure 6).
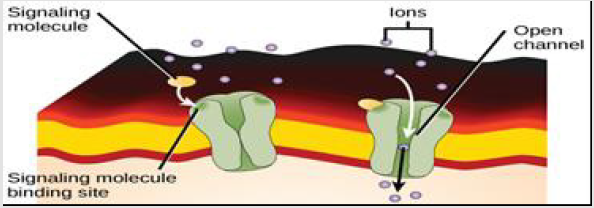
e) Ligand-Gated Ion Channels
When a ligand-gated ion channel receptor binds its ligand, conformation alterations lead to open a cell membrane channel via it ions can pass signals. As, in neural synapse cells, the ions influx through the opening channels lead to potential actions, like the nerve signaling, through depolarizing post-synaptic cells membrane, causing enhancing of voltage-gated ion channels such as Ca2+(a second messenger) [45,46] (Figure 7). There are many other examples of extracellular receptors such as olfactory receptors, adrenergic receptor, epidermal growth factor receptor, insulin receptor, receptor tyrosine kinases, high affinity neurotrophin receptors, fibroblast growth factor receptors, low affinity NGF receptor, ephrin receptors as well as N-Methyl-Daspartic acid (NMDA) receptor.
Intracellular Receptors: Intracellular receptors are soluble proteins localized within their respective areas. They are mainly classified into nuclear receptors and cytoplasmic receptors.
a) Nuclear Receptors
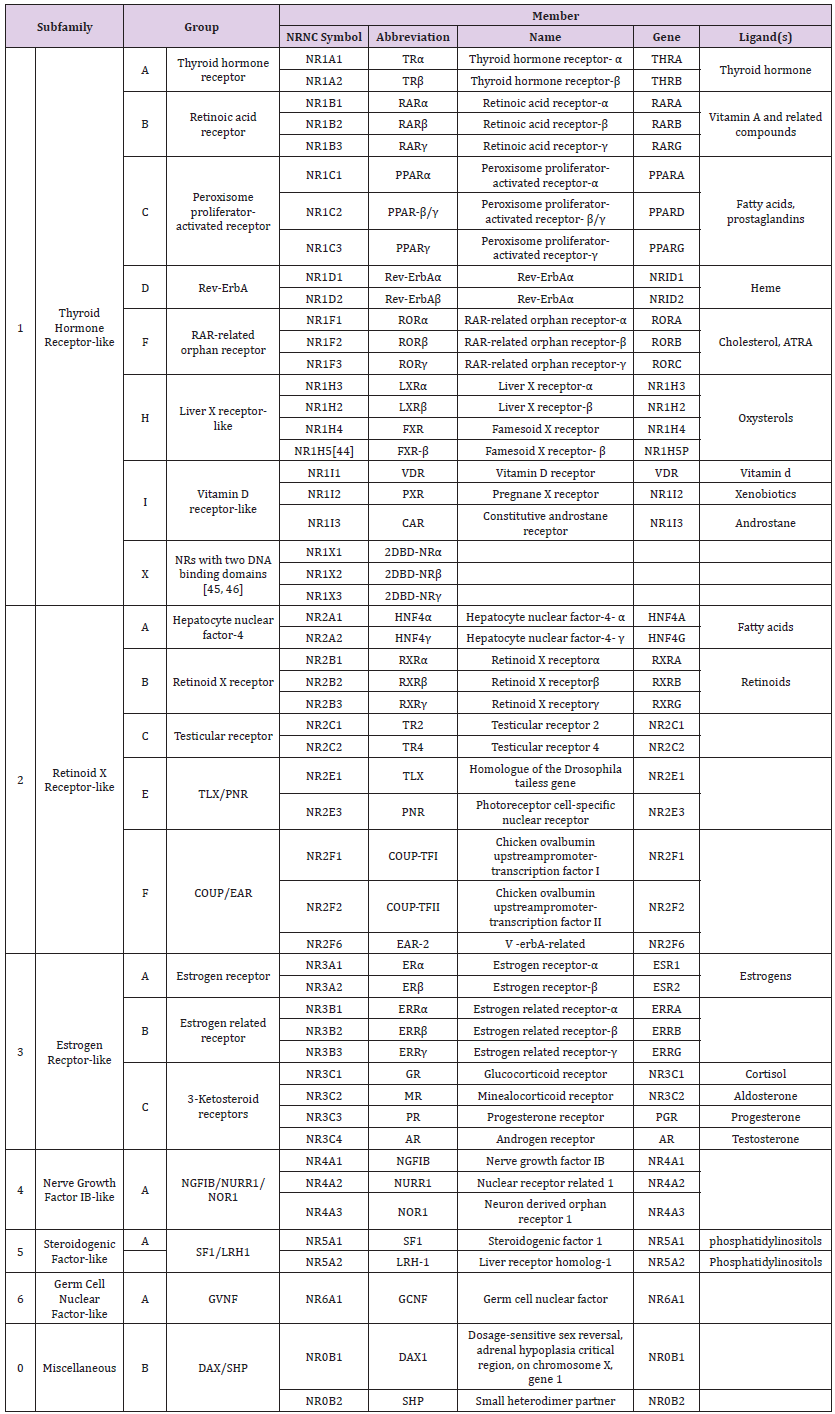
The typical molecular structure of a nuclear receptor is shown in a linear form. All the nuclear receptors contain three main domains (Figure 8) Generally small nuclear receptors are lipid molecules. This property enables it to be diffused through the cellular lipid bilayer as well as the nuclear membrane. The receptor in the nucleus may directly bind to the material of DNA lead to promote the expression. Hormones as the steroid hormones as well as vitamins A and D derivatives, are the ideal ligands for nuclear receptors. In the signal transduction process, the ligand is passively diffused in the plasma membrane, binds its receptors and passes through the nuclear membrane into the nucleus, causing gene expression alteration [47,48]. Activated nuclear receptors bind at receptor-specific Hormone-Responsive Element (HRE) sequences of DNA, found with the genes promoter domain which activated by a complex of hormone-receptor. This process causes gene transcription and its expression. DNA-binding domains of nucleic receptors have zinc fingers as well as a ligand-binding domain; the zinc fingers catalyze binding of DNA through attaching its phosphate backbone. The domain of ligand-binding is responsible for nucleic receptors dimerization before binding as well as constructing structures for transactivation in communication with the translational cases [11] (Table 1).
Table 1: A list of the known human nuclear receptors, classified according to sequence homology [49].
b) Cytoplasmic Receptors
Specific receptors of the intracellular domain of the immune system classified as cytoplasmic receptors.
c) NOD-Like Receptors (NLRs)
The main subclass of cytoplasmic receptors is the NOD-like receptors (NLRs).Recently identified NLRs found in the cell’s cytoplasm and react with ligands by a Leucine-Rich Repeat (LRR) do its response as that of TLRs. The components asNOD2react with receptor-interacting protein kinase 2 (RIPK2)which stimulate nuclear factor kabba B (NF-κB) signaling, whereas others like NACHT, LRR and PYD domains-containing protein 3 (NALP3or cryopyrin) react with inflammatory caspases and start processing of specificcytokinesasinterleukin-1β [49-51].
Second Messenger (Cellular Responses)
Second messengers are molecules that cross the cytoplasm and cause cellular acting to trigger as specific response. Also, they act as biochemical signals from the cellular plasma membrane into the cytoplasm, thus develop the intracellular signal transduction. At a molecular level, the basic mechanisms of responses are modulations in the genes transcription or translation, post-translational as well as conformational changes in proteins, and changes in their location. These mechanisms for controlling cell proliferation, growth, metabolism and other processes [52].The stimulation of many marked pathways through a receptor of singling plasma membrane as well as stimulation of many second messengers through theses effectors may activate a development of signaling transduction, activate diverse, pleiotropic, responses according to the type of the cell [53].
The Classic Cellular Second Messenger Signaling Pathways:
a) cAMP-Dependent Pathway: GPCRs are activated adenylyl cyclase to generate the cAMP. cAMP acts through protein kinase A (PKA, cAMP-dependent protein kinase) activation, subsequent effects relay on cAMP-dependent protein kinase, that differ according to the type of the cell [54].
b) MAPK/ERK Pathway: This pathway related the responses of intracellular signals as growth factors binding to its cell surface receptors [55]. Growth factor receptors activate phosphoinositide 3-kinase (PI3K) to create lipid second messenger phosphatidyl inositol 3,4,5-trisphosphate (PIP3) [53].
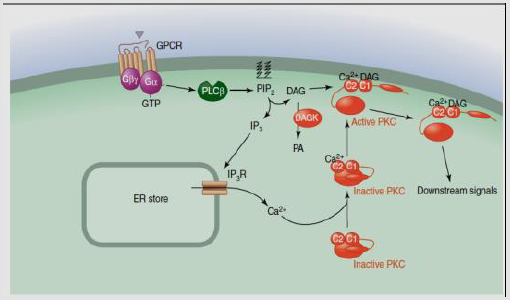
c) P3/DAG Pathway: GPCRs activate phospholipase C (PLC) and cleave the phospholipid phosphatidyl inositol 4,5-bisphosphate (PIP2)to create the membrane-bound messenger diacylglycerol (DAG) which stays linked to the membrane and soluble messenger inositol 1,4,5-trisphosphate(IP3), is liberated in the cytosol. IP3cross the cytosol to link IP3 receptors, especially Endoplasmic Reticulum (ER) Ca channels. Ca specific channels enhance the diffusion of Ca only. This process leads to concentration of the cytosol and elevation of Ca, and alteration of intracellular activity [56]. Also, Ca as well as DAG acts to stimulate Protein kinase C(PKC), which phosphorylate other components, leading to cellular activity alteration [56,53].
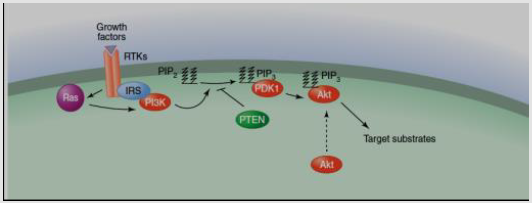
DAG receptor is a small globular domain, C1 domain, identified in PKC [57], as Ras, protein kinase D, DAG kinase, gastrin-releasing polypeptides, as well as n-chimaerin [58]. Activation of PKC isozymes needs the Ca as well as DAG for and leading to signals which stimulate the hydrolysis of PIP2. Transduction signaling via classical as well as PKC isozymes is ultimate through DAG phosphorylation by DAG kinase that ejects the second messenger [59]. For example, PI3K activation following stimulation of receptors of growth factor like insulin receptor creates the phospholipid PIP3, that enrolls the kinases PDK1 as well as membrane Serine/threonine-protein kinases pathway (Akt). The next phosphorylation pathways involve Akt stimulation [53] (Figure 9).
Second Messengers Classes
A. Cyclic Nucleotides: Like cAMP, 3′-5′-cyclic guanosine monophosphate (cGMP) as well as other signaling cytosolic soluble molecules.
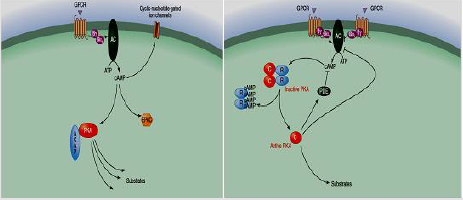
1) cAMP is the major cyclic nucleotides class of second messenger. For example the “fight or flight” mechanism, as the adrenal response. Under extreme stress, epinephrine (adrenaline), from the adrenal gland is stimulated to release into the blood, quickly stimulates impotent physiological as well as cellular interactions which adjust the body for heavy physical activity. Epinephrine links receptors on the cell membrane and activates Adenylyl Cyclases (AC) on the membrane, which catalyzes conversion of AMP to the chemical messenger cAMP, according to the receptor type. The elevations or reduction of cAMP production, that passes through the cellular membrane. The main function of cAMP is to activate phosphorylation of protein [60], finally found its target, cAMP-dependent Protein Kinase (PKA) [61,62]. The small second messenger stimulates PKA at certain domains on the cell due to PKA establishment to A-kinase-anchoring proteins (AKAPs). cAMP activates exchange protein activated by cyclic AMP (EPACs). AC activity is adjusted through the opposing effects of the Gs as well as Gi proteins. The cAMP yielded by AC stimulates PKA via linking to R subunit and out C subunit. These signals may be ultimate through the phosphodiesterase (PDE) enzymesaction [63] (Figure 10).
2) cGMP is another cyclic nucleotide class of a second messenger. It is cGMP composed via receptor of guanylylcyclases, in plasma membrane inner face and is activated through small peptide factors which linked to their extracellular domains. It may be generated by soluble cytoplasmic guanylyl cyclases that are activated via nitric oxide (NO). NO signaling pathway is found in many of vital physiological processes, as neurotransmission and smooth muscle relaxation [65].
B. Lipid Messengers: Are linked with cell membranes signaling. Lipophilic second messenger molecules such as diacylglycerol as well asceramide [66]. Lipid second messenger are involved in many pathways:
a) IP3 and DAG Signaling Pathway
Such as receptors phosphorylation like the EGF as well as receptors of insulin through activation of PKC their internalization as well as development, acting as a negative feedback loop to relieve the signaling by these pathways [53].
b) PIP3 and Akt Signaling
PI3K activation leads subsequent linkage with receptors of growth factor like insulin receptor which stimulates PIP3, activate the PDK1 and Akt to the membrane. The following phosphorylation actions result in Akt stimulation [53] (Figure 11).
c) Sphingolipid Hydrolysis and Protein Targets that Control Apoptosis
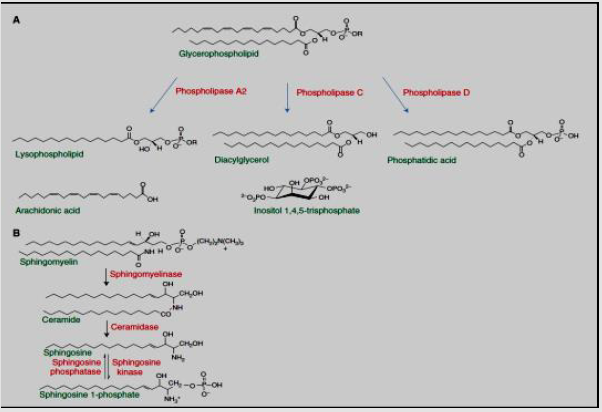
Glycerophospholipids hydrolysis leads to generation of diacylglycerol, lysophospholipids, phosphatidic acid, free fatty acids (arachidonic acid). PIP2 hydrolysis results in water soluble inositol 3,4,5-trisphosphate. On the other handphingolipids hydrolysis results as sphingosine, ceramide, as well as sphingosine 1-phosphate [53]. The main signaling lipids comes from sphingomyelin are ceramide, sphingosine, and sphingosine1- phosphate that are yielded through ceramidase, sphingomyelinase, as well as sphingosine kinase activity respectively. Ceramide stimulates information in the Pathways of stress. Sphingosine possesses apoptotic action. sphingosine 1-phosphate activates the signaling of cellular prosurvival. In case of secretion, it linked to GPCRs class which activates damaged effects on the cell, such as inflammation, differentiation, cell-survival as well as angiogenesis [67]. Intracellular sphingosine1-phosphate functions as a second messenger, activating enzymes like the TNF receptor-associated factor 2 (TRAF2) E3 ligase and some histonedeacetylases [53] (Figure 12).
d) Lysolipids, Prostaglandins and Other Eicosanoids
20-carbon fatty acids oxidation leads to eicosanoids, consequently signaling components which are GPCRs linked activate inflammatory as well as immune responses [68]. Hydrolysis mediated by Phospholipase-A2 of phospholipids composing arachidonic acid in the sn-2 domain of the backbone of glycerol results a fatty-acid compound which may be oxidized via cyclooxygenases to produce prostaglandins, or via lipooxygenases to produce leukotrienes. The produced signaling components linked to certain receptors which bind to diverse pathways. Several of these are yielded in inflammatory responses. For example, leukotrienes linked to GPCRs that produce inflammatory responses often in asthma [69].
Ions as Class of Cellular Second Messenger: Ions like calcium, potassium, magnesium, sodium, chloride, protons, copper as well as iron possess vital cellular function.
a) Potentiate as structural proteins as well as enzymes cofactors.
b) Many cellular operations are susceptible to alterations in their ambient ionic environment, such as pH alteration, may change enzymatic activity as well as the cellular ion transporters behavior [70].
c) Ions are inserted from the extracellular milieu or moved from intracellular stores and manage the protein targets activity by linking to certain motifs on the upstream regulators or protein itself (as calmodulin) [71].
d) Activity of cellular ions manages via electrical signals spreading, such as, heart as well as neurons potentials action.
e) Ions like calcium as well as magnesium can have direct function as dynamic intracellular messengers which adjust certain signal transduction protein targets.
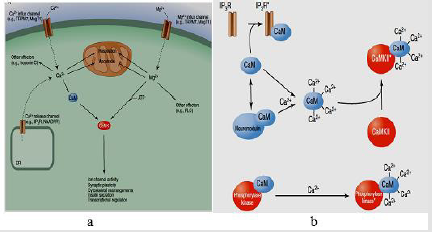
Calcium is an extremely versatile intracellular messenger that manages a broad range of cellular roles through managing the number of target proteins activity. Ca is used in many operations such as contraction of muscle, nerve endings neurotransmitter emission, as well as cell migration. The 3 major pathways that result in its stimulation are GPCR pathways, RTK pathways, as well as ion channels; it directly regulates proteins or via an enzyme linkage [72]. Intracellular Ca concentrations are managed through channels, pumps, transporters as well as buffers assortment and moieties of effector. Ca concentrations are increased via the channels opening either on many organellar stores or in the plasma membrane. Internal stores of Ca liberation present a main source of many Ca cellular signals. Mainly, the major domains of Ca stores are ER, Golgi, sarcoplasmic reticulum (SR) as well as acidic endolysosomal system organelles [73].
Figure 13:a) Calcium and magnesium signals. b) The various ways in which calmodulin can function to alter cellular targets [53].
Other intracellular messenger is magnesium. Mg concentration may dynamically alter due to cellular stimulation [74]. Mg links to nucleotides, oligonucleotides, and large number of enzymes. Of its vital binding sites, ATP. Cellular operations typically use ATP complexed with magnesium as a source of energy. Also, Mg has been documented to produce prolonged prevention of neurons potassium channels after activation of muscarinic acetylcholine receptor, thus adjustment of neuronal excitability [75]. Mg as well as Ca possesses an antagonistic function. Mg frequently prevents the transport as well as cellular activities of Ca and may inhibit many pathological consequences of increases in calcium levels [76] (Figure 13).
Free Radicals (Redox) and Gases Class of Second Messenger: It can signal throughout the cell and to neighboring cells. Nitric oxide (NO) functions as a second messenger because it is a free radical that may cross the plasma membrane and affect nearby cells. It is originated from arginine and oxygen via the NO synthase enzyme and does via soluble guanylyl cyclase stimulation, which when stimulated yields another second messenger, cGMP. NO may act via proteins covalent modification or their metal cofactors; some possess a redox mechanism as well as are reversible. In high concentrations, it has a toxic action and produces damage during stroke. However, it is the cause of various other roles such as blood vessels relaxation, apoptosis, as well as penile erections [77].
Cellular Communication Types
The body communication is subdivided into three different forms which finally stimulate a specific cellular response.
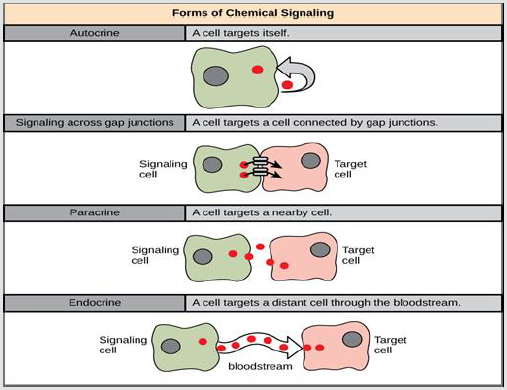
Intracrine Signals
They are activated through the target cell and stay within the target cell (Figure 14).
Juxtacrine Signaling
It is reaction when proteins from the inducing cell binds receptor proteins of neighboring responding cells. Signaling through gap junctions involves signaling components moving directly between neighboring cells. These signals are transmitted along cell membranes through protein or lipid components integral to the membrane and are capable of affecting either the emitting cell or cells immediately neighboring [78].
a) There are three types of juxtacrine interactions.
b) A protein on one cell links to its receptor on the neighboring cell [79].
c) A cell receptor links to its ligand on the extracellular matrix secreted by another cell.
The signal is transmitted directly from the cytoplasm of one cell via small conduits into the cytoplasm of neighboring cell [80,81].
Autocrine Signaling
A signaling where cells may communicate with themselves. In which, the cell directly affects its own function by secreting substances which may act on the cellular receptors. Its signals are generated via the target cell, secreted, and affect the target cell itself by receptors like immune cells [78].
Paracrine Signaling
It is a cell signaling form in which the target cell is near the signal-releasing cell. Some signaling components are very quickly degraded, limited the scope of their effectiveness to the immediate surroundings. Also, only nearby cells are affected because they are taken up quickly, leaving few to travel further, or because their movement is hindered by the extracellular matrix. A paracrin signal eingagents are growth factors and clotting factors are. The local action of growth factor signaling possesses an especially vital function in the development of tissues. Also, cells may communicate with cells in the immediate environment. Paracrine signaling is especially important in the local immune response [80,81].
Endocrine Signaling
Signaling at a distance. Occurs via the presence of hormones. Hormones are biologically active substances which are secreted into the blood stream. Many of the regulators of white cell growth and development, for example, are hormones [80,81].
Egyptian Experiences
In Egypt, cellular signaling pathways were used in many researches like the following: Balbaa [82], has studied the cell signaling and diabetes, he has been investigated an ongoing intensive investigation of insulin-induced signaling molecules in liver and brain tissues during the treatment of streptozotocininduced diabetic rats through combination of Nigella sativaoilin with some anti-diabetic drugs. Balbaa [82], has been confirmed the anti-diabetic action of Nigella sativa oil was via a linked investigation of lipid profile, antioxidant activity and signaling molecules in the absence and presence of some anti-diabetic drugs. Azab, et al. [83] have been studied the glycoprotein H and α4β1 Integrins determine the Entry Pathway of Alpha herpes viruses. Elkholy and Allam [84] Signaling lymphocytic activation molecule family-1 (CD150) expression as a prognostic indicator in patients with chronic lymphocytic leukemia. And have documented that CD150 signaling can be a useful tool in identifying B-CLL patient’s risk.
Conclusion
By understanding the processes that govern these pathways, scientists can understand the flow of information and transmission thereby allowing humans to treat diseases and grow tissues. There are various different ways for cells to communicate with each other and the outside environment. With the advent of computational biology, the analysis of signaling pathways and networks has become an essential tool to understand cellular functions and disease, including signaling rewiring mechanisms underlying responses to acquired drug resistance.
| For more Articles on : https://biomedres01.blogspot.com/ |




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.