A Presentation of a Case of Hb E Homozygosity
Background
Almost a total of 1317 Hb variants have been identified (HbVar
database) [1], the four most common worldwide are Hb S, Hb E, Hb
C, and Hb D, in the order of decreasing prevalence [2]. Haemoglobin
(Hb) E is the most prevalent variant in Southeast Asia (Thailand,
Myanmar, Cambodia, Laos, Vietnam), where its prevalence is 30-
60% [3-6]. The prevalence of HbE in India is about 3.5% with an
increased clustering in Kolkata (22%) and Assam (50-80%) [7]. Hb
E results from a G→A substitution in codon 26 of the β globin gene.
This produces an abnormal Hb (glutamate is replaced by lysine)
and activates a cryptic splice site at codon 25-27 of the β-globin
gene, resulting consequently in abnormal processing for messenger
RNA (mRNA). The level of normally spliced mRNA become reduced
and because a new stop codon is generated, the abnormally spliced
mRNA become nonfunctional [7,8].
Fortunately, only a minor activation of the alternative splicing pathway is associated with this mutation resulting in a moderate reduction of the normally spliced βE globin mRNA 72. Hb E trait (E heterozygosity; ββE) and Hb E disease (E homozygosity; βEβE) are mild disorders. Although the Hb E mutation alone does not cause any significant clinical problems, its interactions with various forms of α and β thalassemia produce a very wide range of clinical syndromes of varying severity [8,9]. Different phenotypes could be noticed with the compound heterozygote state of Hb Eβ-thalassemia ranging from a complete lack of symptoms to transfusion dependency [3,7,8,10]. Experiments were carried out in vitro at temperatures ranging from 38 to 41°C showed that there was a mild instability of Hb E but there is no evidence that this is the case in vivo [9-14]. It is noticeable that the E allele causes a mild thalassemia, while Eβ0 thalassemia shows severe phenotypes. This marked paradox in phenotypes could not be fully explained up till now. It is reported that HbE is sensitive to oxidative stress. Does this or other properties of HbE explain the variable severity of the Eβ0 thalassemia? This question is still waiting for an answer [11]. The aim of this report is to present a case of Hb E homozygosity discovered accidentally trying to cast shadow on a mutation not prevalent in the Middle East.
Case Report
A Consent has been taken from a 26-year-old male, from
Kolkata, India, came to Najran University Hospital, Saudi Arabia for
routine investigation. He did not complain from anemia or receive
treatment. He gave a history of hemolytic attack because of high
fever indifintily due to infection with malaria and only one blood
transfusion. On examination, there were no significant clinical
findings such as organomegaly, icterus, or thalassemic bone changes.
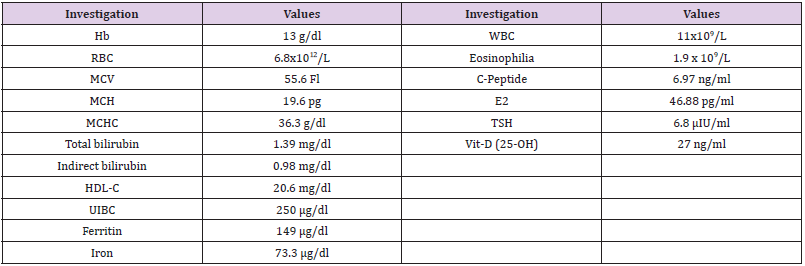
CBC were carried out using Sysmex XS 500i (Sysmex, https://www.
sysmex.com/). It showed mild microcytic hypochromic anaemia with increase in RBC distribution width (RDW-CV) and eosinophilia
(Table 1). Peripheral blood smear showed frequent target cells, and
spherocytes as shown in (Figure 1). Serum biochemical analysis
were carried out using COBAS C311 (Roche, https://www.roche.
com/). Results were normal for liver and kidney functions except
for mild increase in bilirubin (1.39 mg/dL, mostly indirect of 0.98
mg/dL).
Discussion
Hb E has two compartments; one is the Hb E (α2βE2) itself
which behaves like a rather normal Hb in normal conditions. The
other one is the thalassemic compartment with excess α globin
chain leading to a mild haemolytic anaemia .Considering history,
ethnic origin, clinical findings and laboratory findings of the
patient, the diagnosis was consistent with Hb E homozygosity. He
showed a very mild, clinically asymptomatic, hemolytic microcytic
hypochromic anemia with many target cells on peripheral blood
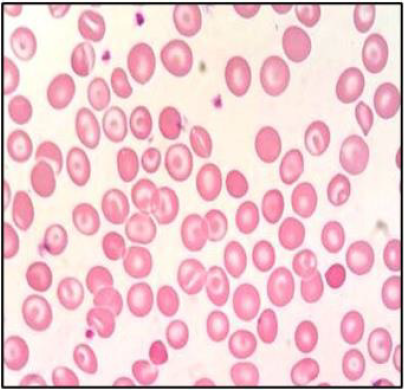
smear [9]. The presence of an abnormal peak of around 85% in
the A2 window with different retention time than Hb A2 (usually
around 3.2 min), a mild increase in Hb F (Figure 2), a mild increase
in the unconjugated bilirubin (Table 1), indicates the mildness
of the pathophysiology of the thalassemic compartment of the
disease. The presence of small peaks on HPLC was explained by
post-translational modification of Hb E13 . Hb E masked the Hb
A2 on HPLC, hense we could not configure its percentage which is
especially important in the diagnosis of δβ thalassemia. We noticed
the presence of many spherocytes in the peripheral blood smear
with an increase of MCHC. Similar findings occur in Hb C due an
increase in the activity of K:Cl- cotransport that induces the loss of
K+ and subsequently of intracellular water [15].
The patient had a low HDL-C, a slightly increased E2 and
TSH hormones, a slightly increased C-peptide which could not be
explained. The patient gave a history of high fever, indifintly due to
malaria infection, with a hemolytic attack and a blood transfusion
once, which could match the published of the instability of Hb E in
high fever [14]. Although investigation for malaria was negative, yet
the presence of eosinophilia (Table 1) may be due to an old infection
[16]. The main differential diagnosis is the Eβ0 thalassemia. Hb E is
usually around 50% with severe clinical presentation and marked
increase in Hb F [17]. Eβ+ thalassemia is milder and characterized
by the presence of Hb A. As there are only two β globin genes, one
on each chromosome, usually the majority of the Hb is abnormal in
case of homozygous β mutation. Other β globin chain variants elute
in the A2 window on HPLC are D-Iran, Osu Christiansborg, Deer
Lodge, G-Coushatta, G-Copenhagen, D-Ouled Rabah, Ocho Rios,
Korle-Bu, G-Ferrara, Zürich and Rocky Mountain, Hb Abruzzo and
M-Saskatoon). Mostly they are non-pathogenic with normal or near
normal clinical presentation. Two fusion gene mutations (Lepore
and Kenya) also elute in the A2 window.
Hb Lepore, a δβ fusion gene is another thalassemic variant result
in δβ thalassemia. In homozygosity, it gives severe thalassaemia
intermedia to thalassaemia major with HbF 80%, Hb Lepore 20%
and no Hb A or Hb A2 [18]. Hb Kenya (considered an HPFH), a
gamma beta (Aγβ) hybrid with expected 22.5 kb deletion is also
expressed at low percentage (6-23 %) with increased Hb F (5-28%)
in heterozygotes. Four alpha globin chain mutations also elute in the
A2 window (Spanish Town, G-Honolulu, Toulon and Fort Worth).
They are expressed at low percentage (11-21% in heterozygous)
usually with no clinical manifestation. As an α-variant, they show the
presence of the characteristic minor Hb A2 variant peak (α2M δ2)
immediately after the α variant peak, which is missing in β variants.
Many other Hb variants (Alabama, Abington, Akron, Bethesda,
Chandigarth, Denver, Ethiopia, G Galveston, G Taipei, Hoshida,
Hamadan, Jeddah, Loves Park, Muravera, Nebraska, San Bruno,
Santa Juana, SId (the aged adduct of Hb S due to glutathione) and
Tubingen) can elute between the A and the A2 windows on HPLC
masking the Hb A2 [19].The majority of hemoglobinopathy present
in the western and eastern provinces of Saudi Arabia, particularly
in the southwestern province. Najran city, in the south of KSA, had
the least prevalence of haemoglobinopathies (β Thalassemia trait
(2.4 %), zero β thalassemia disease, 12.5% sickle carrier and 0.3%
sickle disease). This in contrast to Jazan, also a south city, in which
haemoglobinopathies are clustering. Few cases of Hb E may be
present in Najran and many must be present in Jazan, but there is
no report about the prevelance as far as we know. Orientation by
the different phenotypes by Saudi doctors is important to prevent
misdiagnosis in haemoglobinopathy. Orientation by different
genotypes is critical in premarriage consultation and prenatal
screening programs in the kingdom.
For more Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.