Drugs that Target p53-Mdm2 Interaction
Introduction
The first identified tumor suppressor, p53, is the guardian of the
genome and is tightly controlled by its master negative regulator,
mouse double minute (Mdm2), and its paralogues Mdm4 and
HDMX [1]. p53 is regulated by a negative feedback loop whereby
p53 transcriptionally activates Mdm2. In turn, Mdm2 catalyzes the
ubiquitination and proteasomal degradation of p53. Virtually all
cancers have dysfunctional p53 due to either the gene’s mutation
or to dysregulated p53 signaling in the background of wild-type
p53. The most common mechanism by which p53 signaling is
dysregulated is the overexpression of Mdm2. Its overexpression
occurs in tumors either due to gene amplification or by increased
transcription and or translation. However, it is unusual for Mdm2
overexpression to be combined with mutated p53 in the same tumor.
Consequently, targeting the p53-Mdm2 interaction has become a
desirable approach to developing small molecule anticancer drugs.
Mechanism of Action
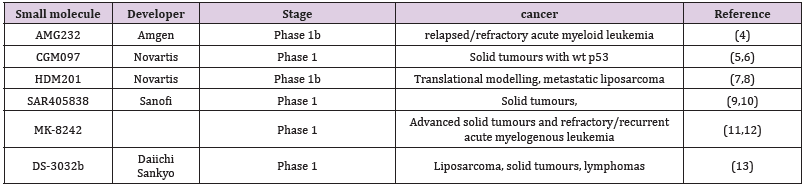
Inhibitors that reactivate the function of p53 act by binding to the Mdm2 p53 binding domain, thereby restoring p53 function inducing cell cycle arrest and apoptosis. This inhibition of Mdm2-p53 binding leads to non-genotoxic p53 stabilization, cell cycle arrest, and apoptosis and is associated with p21WAF1 protein and caspase-3/7 activity [2]. This finding suggests that Mdm2 inhibitors will only function in a p53 wild-type context. The original lead molecule was a p53 peptide that bound snuggly into the hydrophobic pocket in Mdm2 and led to the nutlin 3a, a firstgeneration molecule capable of reactivating p53 function. Nutlin 3a, now known as idasanutlin, as a second-generation molecule, has reached the phase III clinical stages for refractory or relapsed acute myeloid leukaemia when combined with cytarabine [3-5]. Other second-generation molecules currently under development are listed in Table 1 and are many in the first phase of clinical trials [6-9].
Prospects and Limitations of the Strategy
Pre-clinical and clinical trial data show that these Mdm2 inhibitors are well tolerated and can inhibit Hdmx [10-12]. There are better prospects when these drugs are combined with chemotherapy, radiation, or other small molecules [13]. One limitation to using Mdm2 inhibitors is that they ideally compensate for upstream defects in the p53 signaling but may not be effective if there are defects in downstream elements of the pathway. Another shortcoming is that Mdm2 is not the only negative regulator of p53. Indeed there are many negative regulators of p53 [14]. The most critical threat to the development of these drugs is the demonstrated emergence of stable drug resistance, at least in cell culture. In cell culture, these drugs tend to cause cell cycle arrest and little apoptosis. Furthermore, MCF- 7 and U-2 OS cells recovered after the removal of idasanutlin from the culture medium and in de novo generation of p53 mutations was detected in U-2 OS cells after prolonged culture.
Possible Interventions
One of the possible interventions could be the combination of targeted therapies with radiation or with chemotherapy. Pre-clinical studies show that a combination of nutlin 3a and chemotherapy produced additive and synergistic outcomes in cell culture dependent on the effective dose applied (2). The combination therapy with inhibitors of select negative regulators of p53 may be another approach to improve the effectiveness of these drugs. One such is the retinoblastoma binding protein 6 (RBBP6). Like Mdm2 -/- mice, RBBP6 null mutants are embryonic lethal and can be rescued by simultaneous deletion of p53 [15]. Furthermore, this study shows that RBBP6 enhances Mdm2 catalytic activity. Moreover, the RBBP6 Drosophila homolog SNAMA is also embryonic lethal, underscoring that this gene is essential through evolution. Moreover, there has been no Mdm2 homologue found in the fly, and it has been declared that it does not exist [16]. These studies indicate that RBBP6 on its own may accomplish Mdm2 functions in some contexts and that targeting both Mdm2 and RBBP6 is a desirable approach because they are intimately involved in regulating p53. Other potential inhibitors of p53-Mdm2 interactions are those that target the E3 ligase activity. These have, however, proved to be ineffective.
Conclusion
Although this class of compounds has produced promising
results in early-stage clinical studies, the critical concern is the
possible haematological toxicity. However, the poor effectiveness of
these compounds individually to induce apoptosis in cancer cells
is also of great concern. Current studies indicate that combination
therapy could mitigate these shortcomings.
For more Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.