Inflamyar™ Possesses Anti-Inflammatory Effect on Human Immune Cells and Cytokine Expression In Vitro
Introduction
Since virtually all organisms are constantly exposed to the
influences of the living environment, the immune system has a
great importance for the physical integrity of humans. It protects
against threatening external influences such as infestation by
microorganisms and parasites, but also against threats from the
inside of the body, like e.g. necrotic and apoptotic cell material as
well
as functionally degenerated cells [1,2]. The human immune system
is made up of several components. A distinction is made between a
cellular and a humoral part. The cellular immune system comprises
highly specialized immune cells that are either mobile (e.g. in the
blood) or located in various tissues, e.g. Monocytes, Granulocytes,
B cells, T cells, NK cells. The humoral immune system is the part
of the immune system based on plasma proteins (antibodies,
complement factors and cytokines) [2,3]. Upon induction of an
immune response, humoral components of the immune system
are initially released by the cells located in the affected tissue. By
secreting these factors, other immune cells are lured to the focus of
infection [2,3]. The inflammatory response in the body is important for
the resolution of the cause of the inflammation but is also the
cause of the symptoms of the disease [4]. The immune response
is not entirely specific to its cause; even healthy tissue is always
damaged [5].
This is especially important when an acute inflammation becomes a chronic inflammation that does not succeed in elimination of the trigger. For example, this may be due to the presence of debris in the tissue or frequent overload of muscles and joints. This persisting inflammation can massively damage the surrounding, primarily healthy tissue [6,7]. The damage of healthy tissue leads to severe pain reactions in both acute and chronic inflammatory reactions [5,8]. Moreover, chronic inflammation can lead to neoplasms (carcinomas or lymphomas) in many organs and in the lymphatic tissue and promote their growth and vascularization. Causes include the chronic proliferation stimulus, the growth-promoting effects of cytokines and the genomic damage caused by reactive oxygen species produced by immigrated immune cells [9-11]. In such situations, use of anti-inflammatory agents to assist and reduce the side effects of inflammation is useful [12,13]. Among the most widely used anti-inflammatory drugs include i.e., synthetic substances such as cyclooxygenase inhibitors, steroids, immunosuppressants and cytokine inhibitors [14,15]. These substances are usually proven to be extremely effective - but in part also show a wide range of unwanted side effects [16-18]. For this reason, the active ingredients from classical medicinal plants are increasingly becoming the focus of research.
In particular, secondary plant metabolites are in the interest of science since these have been in use in traditional medicine for many centuries and thus relatively safe and side effects associated with their use often relatively low [19-23]. Plant metabolites have a wide range of pharmacological effects, such as high antioxidant, antiviral, anti-inflammatory and carcinogenic activity [24-26]. Antiinflammatory ingredients such as alkaloids, phenols, flavonoids, glycosides, terpenes, quinones, catechins and carbohydrates of aqueous extracts from various traditional herbs have been described in several studies [25,27-29]. Besides of direct antiinflammatory actions, an interaction with immunological signal cascades, such as the reduction of proinflammatory mediators by inhibiting transcription factors of gene expression (Nuclear Factor κB, Inhibitor of κB), has already been demonstrated for some of these substances [30-32]. These modulatory effects of plant substances are particularly interesting for research. The individual effects of active ingredients from plant extracts are very diverse and have so far been described inadequately. For this reason further investigations are mandatory. In this study, the anti-inflammatory effect of Inflamyar™, a commercially available homeopathicspagyric product consisting of plant extracts from Arnica montana, Bryonia cretica, Guajacum, Toxicodendron quercifolium, Bellis perennis, Ledum palustre, Ruta graveolens and Viscum album was evaluated.
Materials and Methods
All experiments were conducted by NIS Labs, Klamath Falls, USA.
Test Substance
The test substance, Flamyar™, is a homeopathic spagyric natural remedy manufactured by PEKANA Naturheilmittel GmbH (Kißlegg, Germany) and distributed in the USA under the name Inflamyar™. The test substance was developed for the treatment of sports injuries, sprains, joint problems, bruises, and muscle strains. Active ingredients are Arnica montana spag. Peka Dil. D12, Bryonia cretica spa. Peka Dil. D4, Guajacum Dil. D4, Toxicodendron quercifolium Dil. D12, Bellis perennis spag. Peka Dil. D8, Ledum palustre Dil. D4, Ruta graveolens spag. Peka Dil. D6, Viscum album spag. Peka Dil. D4.
Reagents
Histopaque1077 and Histopaque1119, RPMI1640, 200mM l-glutamine, antibiotics, fetal bovine serum, BSA (bovine serum albumin), fibronectin, and PBS (phosphate buffered saline) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium azide (NaN3) was acquired from LabChem, Inc. (Pittsburgh, PA, USA). CD3 (peridinin chlorophyll protein), CD25 (brilliant violet 421), CD56 (phycoerythrin) and CD69 (fluorescein isothiocyanate), antibodies as well as heparin vacutainers were ordered from BD Biosciences (Franklin Lakes, NJ, USA). The protein multiplex array (27-Plex human cytokine Bio-Plex Pro™) was obtained from Bio- Rad Laboratories Inc. (Hercules, CA, USA).
CD69 Activation Marker Expression on Human Leukocyte Subsets
Peripheral whole blood from human healthy adult donors (n=3) was obtained upon written informed consent approval by the Sky Lakes Medical Center Institutional Review Board, Federalwide Assurance 2603. Heparinized blood samples were placed on gradient solution (Lympholyte-Poly) and centrifuged at 450 × g for 35 minutes. The layer containing PBMC (peripheral blood mononuclear cells) was separated, washed twice with 10 ml PBS (without Ca / Mg) and resuspended in RPMI 1640 (containing 10% fetal calf serum, l-glutamine and antibiotics (P/S) at a cell density of 106/ml. Two parallel culture conditions were used:
a) Adding of serial dilutions of test product without any other stimuli to test the direct immune modulating effect
b) Adding of serial dilutions of test product, followed by addition of an inflammatory insult in the form of bacterial endotoxin LPS (lipopolysaccharide), to assess the ability of test product to reprogram the human immune cells to respond differently to inflammatory stimuli.
Triple cultures were established for each test condition. Negative controls (untreated cell cultures) were established with n=6. Positive control cultures (2x n=3), n=3 containing 10 ng / ml LPS and n=3 containing 100 IU / ml IL-2 for immune cell activation via two different ways.
After an incubation time of 24 hours at 37°C and 5% CO2, blood cells were isolated and stained for 15 min with fluorochromeconjugated monoclonal antibodies at suppliers recommended concentration and then analysed via Attune acoustic-focusing flow cytometer (Thermo Fisher Scientific). Data analysis was performed using electronic gating based on cell size and granularity to distinguish lymphocytes and monocytes, allowing separate analysis of CD69 expression on lymphocyte subsets opposed to monocyte/ macrophage cell subsets. The subpopulation of lymphocytes was then analysed for CD69 expression on CD3-CD56+ NK cells. Costaining with CD3 and CD56 allowed further detailed analysis of four separate lymphocyte subpopulations, namely CD3-CD56+ NK cells, CD3+ CD56- T lymphocytes, and CD3+CD56+ NKT cells. The combination also allowed us to analyze the CD3-CD56- non-T non-NK lymphocytes for activation markers. For each of these populations we examined the expression level of the CD69 activation marker.
Cytokine Production in Peripheral Blood Mononuclear Cell Cultures
Cell culture supernatants were obtained from 24-hour culture setup described above. Expression levels of the following cytokines were tested: Interleukin (IL)-1β, -1ra, -2, -4, -5, -6, -7, -8, -9, -10, -12 (p70), -13, -15, -17, eotaxin, basic Fibroblast Growth Factor (FGF), Granulocyte-Macrophage Colony Stimulating Factor (GMCSF), Granulocyte-Colony Stimulating Factor (G-CSF), Interferon γ (IFN-γ), interferon γ -Induced Protein 10 (IP-10), Monocyte Chemoattractant Protein 1 (MCP-1), Macrophage Inflammatory Protein (MIP)-1α, MIP-1β, Platelet-Derived Growth Factor (PDGF)- BB, Regulated On Activation, Normal T Cell Expressed And Secreted (RANTES), Tumor Necrosis Factor (TNF)-α, and Vascular Endothelial Growth Factor (VEGF). The assay was analysed using magnetic protein multiplex arrays (BioPlex, Bio-Rad Laboratories Inc.) and xMAP technology (Luminex, Austin, TX, USA).
Statistical Analysis
Calculations and statistical analysis was performed using the two-tailed, independent t-test using Microsoft Excel.
Results
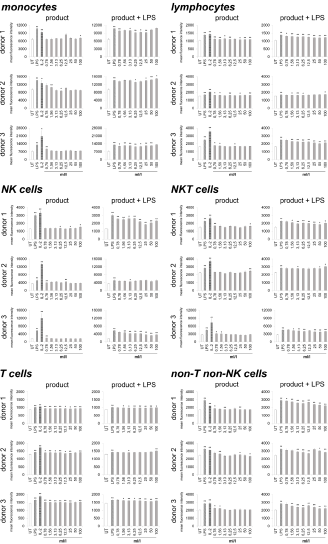
Immune cells harvested from human peripheral blood were used as a model for potential activities on immune activating and modulating. On the one hand, the direct activation of immune cells by the test substance, and on the other hand, the priming of immune cells to respond differently to a subsequent inflammatory insult was analyzed. Therefore, two sets of cell cultures were examined in parallel, on the one hand the highly inflammatory bacterial LPS from Escherichia coli and on the other hand the recombinant Interleukin-2 (IL-2) for activation was used as a positive control, and LPS was additionally used in one of the two cultures to induce inflammation after treating the immune cells with the test substance. As indicator for immune cell activation, the activation marker CD69 (cluster of differentiation 69) was chosen. In the case of lymphoid activation, CD69 is the earliest inducible surface glycoprotein and leads to lymphocyte proliferation and signal transmission at the cellular level [33,34]. Incubation of the cell culture with the test product (Figure 1) led to a slight increase in CD69 expression on monocytes (-6.3 to 28.8%) and lymphocytes (0.8 to 10.2%). Either no effect or else a slight increase was seen on Natural Killer (NK) cells (-4.1 to 24.7%), Natural Killer T (NKT) cells (-6 to 14.3%), non-T non-NK lymphocytes (-4.3 to 12.5%) and T cells (2.8 to 8.6%).
Figure 1: CD69 expression on immune cells in 24-hour cultures of peripheral blood mononuclear cells treated with products alone (column “product”) or pretreated with product prior to the addition of the inflammatory insult LPS (column “product + LPS”) plotted as CD69 mean fluorescence intensity. Statistical significance is indicated on the bar graph (*p<0.05, **p<0.01).
Under inflammatory conditions, treatment of cultures with the test product led to a reduced CD69 expression in monocytes of all three donors at lower concentrations of the test product (-0.4 to 14.4%). The highest concentrations of the test substance showed an induced expression of CD69 up to 5.9%. The lymphocytes possessed a reduction of CD69 expression from -2.7 to 19.19% for all three donors. A 38.6 to 42.0% reduction was seen in NK cell activation for both donor 1 and donor 3; donor 2 also showed a reduction in NK cell activation but the response was more variable (ranging from -1.1 to 12.0%). Incubation of NKT cells with the test product prior to LPS stimulation resulted in a -6.3 to 19.2% reduction of CD69 expression for all three donors. T cells possessed a slight reduction in CD69 expression for both donor 1 and donor 3 (-0.2 to 5.3%); donor 2 showed a slight increase in T cell activation (5.3%) at the highest dose tested with no change at lower concentrations of the test product. CD69 expression in non-T non-NK lymphocytes showed a 2.0 to 24.0% reduction in CD69 expression for all three donors. A dose-dependency was seen for donor 1 and donor 3 on NK cells, NKT cells and lymphocytes; on T cells only for donor 3 and for all donors in non-T non-NK cells.
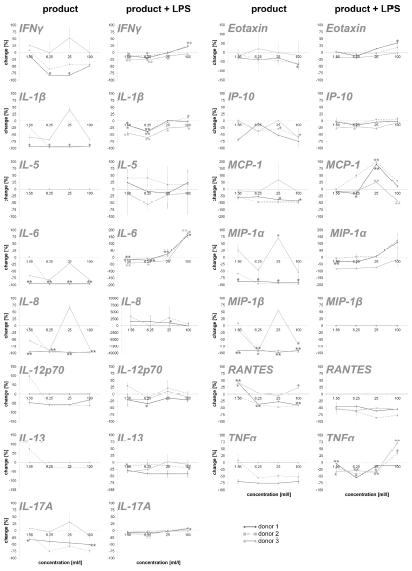
To obtain an overview of the effects of the test substance on the humoral components of the immune system, the test substanceinduced cytokine expression changes were analyzed on PBMC. In this part of the study, on the one hand, the direct effect of the test substance on PBMC cultures and, on the other hand, the effect of pre-incubation of PBMCs with the test substance and subsequent inflammatory stimulus (LPS) were tested. The exposure of immune cell cultures to the test substance without a subsequent inflammatory stimulus (LPS) resulted in a reduction in the cytokine IL-6 up to 98.9% (Figure 2). For two out of three donors (donor 1 and donor 2) the exposure of immune cell cultures to the test product resulted in consistent decreases in the following proinflammatory cytokines: INF-γ (8.5 – 82.2%), IL-1β (21.6 – 97.5%), IL-8 (8.6 –98.4%), IL-12p70 (46.5 – 61.4%; except lowest doses on cells of donor 2), IL-17A (12.0 – 75.7%), Eotaxin (28.4 – 65.4%; except lowest doses on cells of donor 2), IP-10 (3.9 – 74.7%), MCP-1 (55.0 – 97.7%), MIP-1α (58.8 – 97.3%), MIP-1β (13.8 – 97.2%; except lowest doses on cells of donor 2), RANTES (9.1 – 47.6%; in donor 1 and 2) and TNFα (52.4 – 75.6% in donor 1 and 2). The exposure of PBMC cultures to the test substance resulted in a reduction in the cytokine IL-13 in cultures from one donor (29.6%, without lowest dose) and no change in cultures from the other two donors. No change was seen for the cytokine IL-5 in PBMCs exposed to the test substance.
Figure 2: Percent change in proinflammatory cytokine levels in PBMC culture supernatants of three donors treated with serial dilutions of the test product in absence (column “product”) and presence (column “product + LPS”) of a subsequent inflammatory stimulus. Samples without inflammatory stimulus are compared to untreated control cultures; samples with inflammatory stimulus are compared to LPS-control (*p<0.05; **p<0.005).
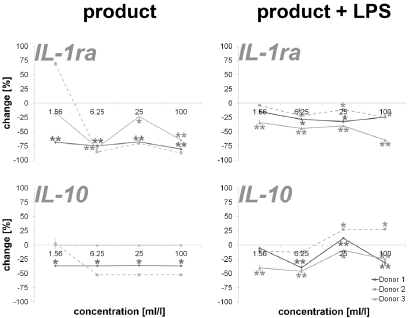
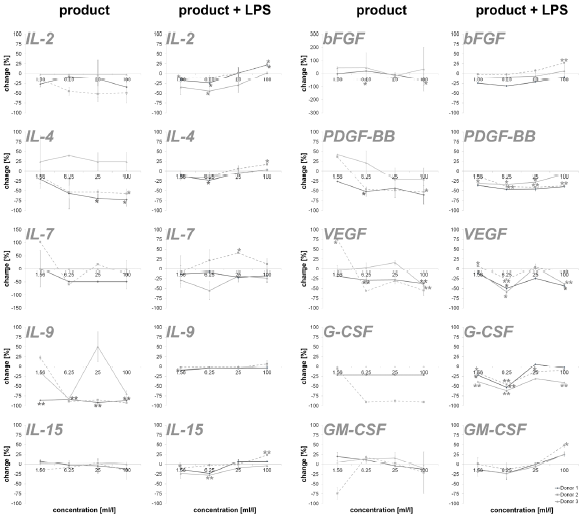
An incubation of PBMCs with the test substance resulted in a reduction of the anti-inflammatory cytokines IL-1ra (16.2 – 87.4%; except lowest doses on cells of donor 2) and IL-10 (35.8 – 52.2%; except lowest doses on cells of donor 2). The data is shown in Figure 3. Measuring cytokines with pro- and anti-inflammatory properties (Figure 4), the following reactions could be assessed: For two out of three donors (donor 1 and donor 2) the exposure of immune cell cultures to the test substance resulted in consistent decreases in IL-2 (8.6 – 51.9%), IL-4 (13.0 – 72.8%), IL-9 (85.1 – 92.5%; except lowest doses on cells of donor 2) and IL-15 (-8.0 – 16.2%) levels. The exposure of immune cell cultures to the test substance led to variable effects on IL-7 (-102.9 – 60.0%) levels. In the group of growth factors (Figure 4), for two out of three donors (donor 1 and donor 2) the exposure of PBMCs to the three highest doses of the test substance resulted in consistent decreases in PDGF-BB (26.2 – 60.3%; except lowest doses on cells of donor 2), VEGF (18.9 – 56.2%; except lowest doses on cells of donor 2) and G-CSF (18.9 – 56.2%; except lowest doses on cells of donor 2). Variable effects were detected in bFGF (-44.9 – 55.4%) and GM-CSF (-20.0 – 74.1%) levels. In total, a clear dose-dependency of cytokine expression could be seen in IL-17A, Eotaxin MCP-1, RANTES, IL-1ra, IL-4, IL- 15, bFGF, PDGF-BB, VEGF and GM-CSF in a minimum of two donors.
The exposure of PBMCs to the test substance with a subsequent inflammatory stimulus (LPS) led to consistent decreases in IL-13 (2.4 – 34.9%) and RANTES (43.8 – 85.7%). An incubation with the three lowest doses of the test substance led to consistent decreases in the following cytokines: INF-γ (0.0 – 26.3%), IL-1β (-1.4 – 41.4%), IL-17A (-9.1 – 7.8), IP-10 (-6.4 – 27.6%) and TNF-α (4.2 – 41.4; except highest doses on cells of donor 2 and 3). A consistent biphasic response, i.e. increases at higher doses and decreases at lower doses of the test substance, was seen for IL-6 (-177.4 – 19.3%) and Eotaxin (-35.2 – 12.8%). Variable effects were detected on IL-5 (-40.8 – 55.4%), IL-8 (-3356.6 – 79.8%), IL-12p70 (-97.6 - 58.8%), MCP-1 (-98.8 – 42.6%) and MIP-1α (-123.2 – 79.3 %) levels and no effects were seen on MIP-1β levels (Figure 2). Consistent decreases in IL-1ra (3.9 – 64.7%) and variable effects on IL-10 (-27-6 – 45.8%) levels were detected in PBMC cultures (Figure 3). The exposure of immune cell cultures to the three lower doses of the test substance led to consistent decreases in the following cytokines: IL-2 (12.0 – 44.5%), IL-4 (9.1 – 23.3%), IL-9 (1.8 – 10.2%) and IL-15 (2.8 – 27.9%); variable effects were found on IL-7 levels (-40.7 – 29.0%).
Under inflammatory conditions, the exposure of PBMC cultures to the test substance led to a decreased concentration of bFGF (9.3 – 24.4%; except donor 2), PDGF-BB (7.8 – 46.3%) and GM-CSF (-0.3 – 23.0%; except highest dose) in a concentration dependent manner. The growth factors VEGF (-7.5 – 58.8%) and G-CSF (-5.9 – 61.6%) showed variable results with a decrease in cytokine expression in two of three donors. According to the results from stimulation of PBMC without inflammatory stimulus, very consistent responses were seen for all three donors (Figure 4). Also in this group, a dosedependent cytokine expression was found in IFN-γ, IL-1β, IL-6, IL- 17A, Eotaxin, IP-10, MIP-1α, TNFα, IL-2, IL-4, IL-9, IL-15, PDGF-BB, GM-CSF and IL-10.
Figure 3: Percent change in anti-inflammatory cytokine levels in PBMC culture supernatants of three donors treated with serial dilutions of the test product in absence (column “product”) and presence (column “product + LPS”) of a subsequent inflammatory stimulus. Samples without inflammatory stimulus are compared to untreated control cultures; samples with inflammatory stimulus are compared to LPS-control (*p<0.05; **p<0.005).
Figure 4: Percent change in levels of cytokine with pro- and anti-inflammatory capacities and growth factors in PBMC culture supernatants of three donors treated with serial dilutions of the test product in absence (column “product”) and presence (column “product + LPS”) of a subsequent inflammatory stimulus. Samples without inflammatory stimulus are compared to untreated control cultures; samples with inflammatory stimulus are compared to LPS-control (*p<0.05; **p<0.005).
Discussion
In the present study, stimulation of immune cells with the test substance was done in the presence and absence of a subsequent inflammatory stimulus. Thereafter, CD69 expression, i.e., the activation of immune cells, was measured along with the cytokine expression. The data show no effects or only a slight induction of CD69 expression after stimulation of the immune cells with the test substance in the absence of an inflammatory stimulus. With the exception of the T cells, the immune cells showed only isolated inductions of CD69 expression compared to untreated controls. These inductions were distributed over the entire concentration spectrum of the test substance and no dose-dependency was seen. In addition, a reduced expression of proinflammatory cytokines was measured in groups without subsequent inflammatory insult. Noteworthy here is the particularly strong reduction of IL-1β, IL-6, IL-8, MIP-1α, MIP-1β and TNFα. The anti-inflammatory cytokines and growth factors also show a regulation in this context; in relation to the proinflammatory cytokines, however, this is more moderate. This suggests a shift of pro- and anti-inflammatory cytokines activity ratio towards an anti-inflammatory level. Together, the findings from CD69 and cytokine expression indicate an antiinflammatory effect of the test substance.
Upon incubation of immune cells in the presence of a subsequent inflammatory stimulus, a reduction in immune cell activation could be demonstrated for most of the samples in two out of three donors, indicating an anti-inflammatory effect of the test substance also under inflammatory conditions. Correlatively, a reduced expression of cytokines and growth factors the doses of 1.56 to 25 ml/l of test substance was measured. Interestingly, the results show an induction of cytokine expression in a concentration range of 25 to 100 ml/l of test substance, in particular of proinflammatory cytokines. This biphasic effect of the test substance (cytokine reduction at low levels and induction at high concentrations) is in contrast to the reduction of CD69 expression at this concentration. An association between reduced CD69 expression at high doses of the test substance and increased cytokine expression at the same dose, in addition to the shift in the balance between pro- and anti-inflammatory cytokines seen in the previous experiments, indicates an additional anti-inflammatory mechanism of action of the test substance. However, further investigations are needed to verify this.
Unfortunately, not all donors in this study showed an equal response to the test substance. In view of the individual variations in the immune status of the donors (e. g. genetic and epigenetic aspects, potential pre-existing conditions), high deviations in the data from primary cells of different donors are not unexpected [35- 37]. From this point of view, the results of this study are relatively consistent. The observed dose-dependent regulation of the CD69 and cytokine expression of individual groups clearly underlines the validity of the data. In addition, the data generated in this study is consistent with previously published data from other workgroups. For example, a significantly reduced in vitro LPS-induced expression of TNFα, IL-1 and IL-6 from the human whole blood culture and RAW-264.7 cells showed Mahajan et al. after incubation with different dilutions of A. montana and Bryonia species (6CH, 30CH, 200CH) [38]. Lussignoli et al. showed a significant reduction in systemic IL-6 expression in a traumatic animal model after the use of a homeopathic preparation containing A. montana and other plant extracts and minerals [39]. In another study, T. quercifolium in dilutions of 6CH, 12CH, 30CH and 200CH appeared to interfere with an histamine, prostaglandins and other inflammatory mediators driven inflammatory processes [40]. Anti-inflammatory actions due to inhibition of both lipoxygenase and cyclooxygenase metabolic pathways were also seen in homeopathic remedy containing A. montana and T. quercifolium [41]. Porozov et al. described reduced IL-1β, TNFα and IL-8 secretion without an effect on human T cell and monocyte proliferation by a homeopathic remedy containing A. montana and B. perennis [42]. In addition, various studies showed anti-inflammatory properties of various extracts out of A. montana [43-48], Bryonia species [48-50], T. quercifolium [40,51,52], B. perennis [53], L. palustre [54,55], R. graveolens [56-60] and V. album [61-65], which are also contained in the test substance of this study. In summary, the data of the present study possesses a clear antiinflammatory effect and thus a potential for the test substance for the treatment of acute or chronic inflammatory reactions.
For more Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.